




Learn the d and f Block Elements in the Periodic Table
The periodic table includes elements that fall into two distinct categories based on the filling of their orbitals: the d-block and the f-block elements. These blocks consist of metals that exhibit unique chemical properties and serve critical roles in various applications.
What are d and f Block Elements?
The d and f block elements are those where the d and f orbitals progressively fill as you move through the periods. The d-block elements, found in groups 3 to 12, are commonly known as transition elements. The f-block elements, which are typically located at the bottom of the periodic table, consist of lanthanides and actinides, where electrons fill the 4f and 5f orbitals.
Applications of d-Block Elements
Many d-block elements, such as iron and zinc, are of significant industrial and economic importance.
Iron
Iron is extensively used in the construction industry, forming the basis of steel. It is crucial in machinery, vehicles, and structural elements like bridges. Iron's alloys are used for their strength and versatility, and it also plays a key role in producing ammonia through the Haber process.
Zinc
Zinc serves as an important anode in batteries and is used to galvanize metals, protecting them from corrosion. It is vital in the automotive industry, electrical components, and even in cosmetic and pharmaceutical products.
Group 13 Elements
The p-block of the periodic table begins with the Group 13 elements. These elements include:
Boron (B)
Aluminium (Al)
Gallium (Ga)
Indium (In)
Thallium (Tl)
Nihonium (Nh)
Boron is a typical non-metal in the Group 13 family. Aluminium, however, is a metallic element that shares similar properties with Boron. Gallium, Indium, and Thallium exhibit metallic characteristics. Collectively, these elements are often referred to as Boron group elements.
d-Orbital Chemistry
The d-block elements' unique electronic properties arise from their partially filled d-orbitals. This configuration leads to distinctive magnetic, catalytic, and bonding behaviours. Recent studies have shown that alkaline earth metal complexes can exhibit similar electronic properties to transition metal complexes, indicating that even elements outside the d-block can participate in similar bonding interactions.
f-Block Elements
The f-block elements include two series: the lanthanides (elements 57 to 71) and the actinides (elements 89 to 103). These elements are known for their powerful reducing properties and are involved in various applications, especially in nuclear technology. Lanthanides are typically non-radioactive, except for promethium, while actinides are mostly radioactive.
H2- Properties of f-block Elements
Both lanthanides and actinides are known for their high reactivity and are typically found in compounds that act as reducing agents. Their chemistry is dominated by their ability to lose electrons easily and form strong bonds.
Classification of F Block Elements
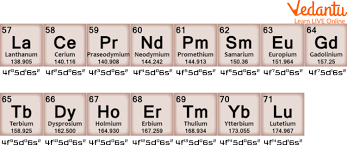
Lanthanide Series
The lanthanides are essential in industries requiring strong magnetic properties and are used in catalysts and high-performance materials.
Actinide Series
Actinides are largely radioactive and include elements used in nuclear reactors, providing key insights into both energy production and nuclear chemistry.

Key Differences Between d-block and f-block Elements
Practice MCQs on d and f Block Elements
1. Which of the following is a characteristic property of transition metals?
A) High ionization energy
B) Multiple oxidation states
C) Non-conductivity
D) Low melting points
Answer: B) Multiple oxidation states
2. Which of the following is not a lanthanide element?
A) Neodymium
B) Uranium
C) Cerium
D) Lanthanum
Answer: B) Uranium
3. What is the primary use of actinide elements like Uranium?
A) Making permanent magnets
B) Nuclear energy production
C) Color pigments
D) Alloy formation
Answer: B) Nuclear energy production
4. Which of the following elements is most commonly used as a catalyst in catalytic converters?
A) Iron
B) Zinc
C) Platinum
D) Nickel
Answer: C) Platinum
5. Which d-block element is commonly used for electrical wiring due to its high conductivity?
A) Zinc
B) Copper
C) Iron
D) Silver
Answer: B) Copper
6. Which of the following statements about the actinide series is true?
A) All actinides are radioactive
B) Actinides have filled 5f orbitals
C) Actinides are found in the f-block section of the periodic table
D) Actinides are non-reactive
Answer: A) All actinides are radioactive
D and F block elements are important for understanding chemistry in NEET. The d-block elements are known for their multiple oxidation states and catalytic properties. The f-block elements, including lanthanides and actinides, are crucial in fields like nuclear energy. Knowing their properties and uses will help in NEET preparation.
Essential Study Materials for NEET UG Success
Glycolysis is a fundamental metabolic pathway present in all living organisms. It serves as the first step of cellular respiration, breaking down glucose into pyruvate and generating ATP. This process occurs in the cytoplasm and does not require oxygen, making it a universal pathway in both aerobic and anaerobic conditions.
Why is Glycolysis Important?
Glycolysis plays a vital role in cellular metabolism and energy production. Its significance includes-
Energy Production- Generates ATP, the energy currency of cells, essential for cellular functions.
Universal Metabolic Pathway- Occurs in both prokaryotic and eukaryotic cells.
Oxygen-Independent Process- Can function under anaerobic conditions, ensuring survival in low-oxygen environments.
Interconnection with Other Metabolic Pathways- Glycolysis provides intermediates for pathways like gluconeogenesis, fatty acid synthesis, fermentation, and the pentose phosphate pathway.
Glycolysis- Process and Importance
The metabolic pathway of glycolysis involves the oxidative breakdown of one glucose molecule into two pyruvates by capturing some amount of ATP and NADH. Glycolysis is the common pathway that happens in both aerobic and anaerobic respiration.
Glucose is the only source of energy that is supplied to the brain, to function the brain properly the body must supply a sufficient amount of glucose to the brain via blood.
Glycolysis- Pathway Energetics and Significance
Glycolysis occurs in both the prokaryotes and eukaryotes. Even though there are different mechanisms that happen in the body, glycolysis is the most important one as it produces the intermediate that is required for other metabolic processes. The glycolysis process occurs in the cytosol and it is a very important process in organisms that do not contain mitochondria. The end product of glycolysis is pyruvate, which acts as an intermediate of various pathways such as gluconeogenesis, fermentation, etc.
The energetics of glycolysis include, from one glucose molecule, two molecules of glyceraldehyde 3-phosphate are formed in the second stage of glycolysis from which, the two molecules of pyruvate are obtained as end products of glycolysis. Hence the energy of glycolysis is calculated by considering two molecules of glyceraldehyde 3-phosphate.
Anaerobic Glycolysis and Its Energetics
Anaerobic glycolysis occurs in the absence of oxygen, during insufficiency of oxygen, and in case of the high demand of energy in the muscles, the anaerobic glycolysis pathway occurs. As the RBCs lack mitochondria, they derive energy from lactic acid fermentation. Another example where anaerobic respiration takes place is in the lens of the eye.
Two processes occur under anaerobic glycolysis, they are-
Lactic Acid Fermentation- This process occurs in the absence of oxygen in the muscles where lactate is converted into pyruvate with the help of an enzyme called lactate dehydrogenase.
Ethanol Fermentation- In this process, glucose gets converted into ethanol instead of pyruvate.
Hence we can tell that the end product of anaerobic respiration is lactic acid or ethanol along with the ATP molecules.
The Role of Glycolysis in Evolution
The evolutionary significance of glycolysis includes-
Ancient prokaryotes used glycolysis before oxygen was present in the atmosphere.
Bacteria produced O2 exclusively from photosynthesis → prokaryotes generate ATP exclusively from glycolysis.
Glycolysis is the most widespread metabolic pathway and does not require any membrane-enclosed organelles.
Interconnection of Glycolysis with Other Metabolic Pathways
Glycolysis serves as a central hub connecting multiple pathways-
Gluconeogenesis- Reverse of glycolysis, converting pyruvate back to glucose.
Fatty Acid Synthesis- Acetyl-CoA derived from pyruvate fuels lipid synthesis.
Amino Acid Biosynthesis- Glycolysis intermediates help form alanine and other amino acids.
Pentose Phosphate Pathway- Provides precursors for nucleotide and amino acid synthesis.
Gluconeogenesis Significance
Gluconeogenesis is a pathway that consists of a series of eleven enzyme-catalyzed reactions. The pathway will begin in either the liver or kidney, in the mitochondria or cytoplasm of those cells, it is dependent on the substrate used.
The gluconeogenesis significance is as follow-
When sufficient amounts of carbohydrates are not obtained from diet this process provides the required glucose.
By the process of glycogenolysis, the glycogen stored in the adipose tissue and skeletal muscle is converted into glucose.
It is used to clear the products of the metabolism of other tissues from the blood.
Energetics of Gluconeogenesis
Gluconeogenesis is an energy-intensive process that synthesizes glucose from non-carbohydrate sources. It primarily occurs in the liver and requires six ATP equivalents per glucose molecule. The key irreversible steps of glycolysis are bypassed using enzymes like pyruvate carboxylase, PEP carboxykinase, fructose-1,6-bisphosphatase, and glucose-6-phosphatase. The process ensures a continuous glucose supply during fasting or intense exercise.
Biological and Evolutionary Significance of Glycolysis
Ancient Metabolic Pathway- Glycolysis is one of the most primitive metabolic pathways, indicating its evolution long before the presence of oxygen on Earth.
Survival Mechanism- Cells that lack mitochondria (e.g., red blood cells, eye lens cells) rely solely on glycolysis for energy.
High Energy Demand Adaptation- During intense muscle activity, when oxygen supply is low, anaerobic glycolysis provides quick energy.
Glycolysis is a vital energy-producing pathway that supports all forms of life, from single-celled bacteria to complex multicellular organisms. Its flexibility in functioning both aerobically and anaerobically ensures cellular survival in diverse environments. Moreover, its integration with other metabolic pathways highlights its central role in maintaining life processes.
Essential Study Materials for NEET UG Success
D and F Block Elements

 Share
ShareFAQs on D and F Block Elements
1. How many f-block elements are present in the periodic table?
There are 28 f-block elements in the periodic table. These elements are divided into two groups: the lanthanides and the actinides. Each group has 14 elements. The lanthanides are elements 57 to 71, and the actinides are elements 89 to 103. Together, they make up the f-block elements.
2. What is the difference between D-block and F-block elements?
The main difference between D-block and F-block elements is the type of orbitals that their electrons occupy. D-block elements fill their d-orbitals, while F-block elements fill their f-orbitals. D-block elements are also known as transition metals and have variable oxidation states. F-block elements include lanthanides and actinides, often involved in nuclear reactions. Both types of elements have important industrial applications due to their unique properties.
3. What are the properties of d and f block elements?
d block elements are usually solid at room temperature and can be either paramagnetic or diamagnetic. They are mostly stable and commonly found in the Earth's crust. f block elements are also solid but are mostly paramagnetic and many of them are radioactive. These elements are rare in nature compared to d-block elements. While d-block elements are more abundant, f-block elements are less commonly found in the Earth's crust.
4. Why is lanthanum considered part of the f-block?
Lanthanum is placed in the f-block because it shares similar properties with other f-block elements. Even though its electron configuration might suggest it belongs to a different block, its chemical behaviour aligns with that of the lanthanides. This makes it appropriate for inclusion in the f-block. Its characteristics are more like those of the f-block elements than the d-block ones.
5. Why do d-block elements have colours?
D-block elements are coloured because they have unpaired electrons in their d-orbitals. These unpaired electrons absorb certain light wavelengths, causing the d-orbitals to split into different energy levels. The light that passes through then has a colour opposite to the one absorbed. This is called a d-d transition. This is why d-block elements show different colours.
6. What is the valency of d and f-block elements?
The valency of d and f-block elements depends on both their valence electrons and the electrons in their d and f orbitals. Generally, the valency of these elements is 2 or 3. However, this can vary based on the specific element and its electron configuration. For many transition metals, valency can change depending on the chemical reactions they undergo. Understanding the electron structure is key to determining the exact valency.
7. What makes f-block elements special?
f-block elements have high melting and boiling points. They can show different oxidation states and form colourful ions. These elements are also known to form complex compounds. Their unique properties make them important in many chemical reactions.
8. Why are d-block elements called transition metals?
D-block elements are called transition metals because they are located between the s-block and p-block elements in the periodic table. These metals show properties that are a mix of both s-block and p-block elements, making them transition between the two. They are also known for their unstable nature and varying oxidation states.




















 Watch Video
Watch Video


