




How to Find Germanium’s Valence Electrons and Their Significance
Germanium is the third element that belongs to the periodic table's fourteenth column. It is classified under the metalloid group. The properties of the germanium element are similar to that of the other metalloids present in the periodic table, such as silicon and arsenic. There are 32 electrons and 32 protons in the germanium atoms with 4 valence electrons in its outer shell.
Clemens Winkler discovered the germanium element in the year 1886 in Germany. The word germanium derived its name from the word Germania, the Latin name for Germany. In this article, we are going to know about the important facts, applications and periodic table of germanium.
Important Facts about Germanium
Under certain standard conditions, the germanium element is a hard, shiny, silvery-grey solid with very brittleness. It is one of those few elements that expand when they are frozen or turn solid.
Since germanium is a metalloid, it consists of both qualities, i.e., metal and non-metal. Germanium has the electrical conductivity between an insulator and a conductor; therefore, it is also called a semiconductor. This characteristic of germanium has made it useful in the electronics field.
At room temperature, germanium does not react with oxygen but will form germanium dioxide when undergoing higher temperatures.
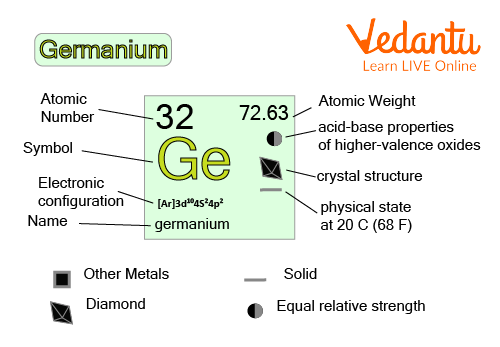
Image Showing Different Properties of Germanium Elements
Quick Recap!
Now let's see how focused you were while reading the above sections of the article. So answer the question. How many valence electrons does germanium have?
Yes, you answered correctly; germanium has 4 valence electrons in its outermost shell.
What Are Valence Electrons?
Valence electrons are the outermost, or highest-energy electrons in an atom. The number of valence electrons defines what kind of element it is. They can participate in bonding and are essential to chemical bonds which hold atoms together in molecules.
The electronic configuration of Germanium is 1s2 2s2 2p6 3s2 3p2 3d10. This shows that to complete the last shell, germanium has 4 less electrons. Therefore, the valence electrons of Germanium is 4.
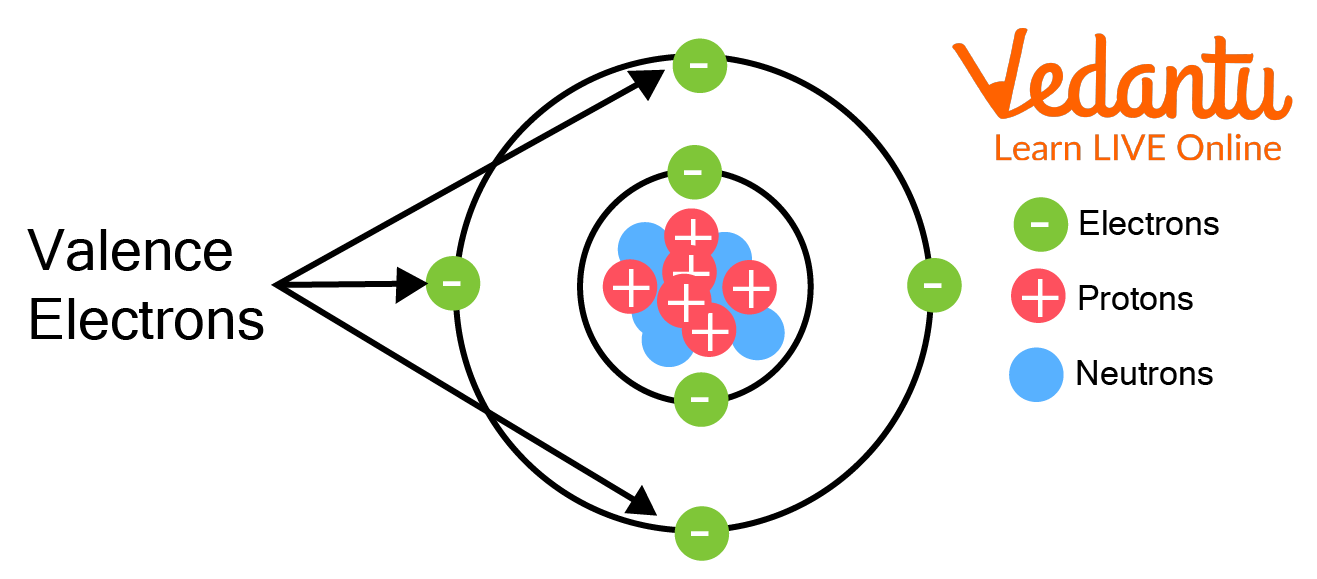
An Atom
The Germanium Element
Germanium is an extremely rare element that is found in the earth's crust. Although some minerals contain a fair amount of germanium, such elements are germanite and argyrodite. These all are too rare to be mined. From the germanium used by the industries, the majority of it is produced as a byproduct of mining the compound sphalerite zinc ore, where it is found in very small traces.
Germanium has quite similar properties to silicon. Germanium is stable in air and water and is not affected by any alkalis and acids, except for nitric acid. Now so many other substances are also used as semiconductors. Still, germanium remains of primary importance in the manufacture of transistors and components for the devices like rectifiers and photocells.

Image Showing What Germanium Looks Like
Summary
From Winkler's home country Germany, the element germanium got its name. Germanium has a total of five naturally occurring isotopes. The most common form of those five is Germanium-74. Germanium atoms have 32 electrons with a shell structure of 2.8. 18.4.
As we know, germanium is a semiconductor, this pure element was commonly doped with arsenic, gallium or some other elements and used as a transistor in several electronic applications. However, today many other semiconductors have replaced it. Germanium oxide shows a high index of refraction and dispersion. The germanium element is a very pure metal which has low density. It is then advantageous for electronic applications and semiconductors.
FAQs on Valence Electrons of Germanium: Complete Guide
1. Enlists the uses of germanium.
Germanium dioxide is also used in the catalysts for polymerisation in the production process of polyethylene terephthalate (PET). The high brilliance of this polyester is especially favoured for the polyethylene terephthalate (PET) bottles marketed in Japan. In the United States, germanium is not used for polymerisation catalysts.
The major end uses for the germanium are:
It is used:
As 35% of fibre-optics
30% as the infrared optics
15% as the polymerisation catalysts
15% as the electronics and solar electric applications
The remaining 5% is used as phosphors, metallurgy, and chemotherapy
2. Write interesting facts about Germanium.
The germanium element has the following:
Symbol: Germanium is represented with the symbol Ge.
Atomic Number: The atomic number of the germanium element is 32.
Atomic Weight: Germanium has an atomic weight of 72.64.
Classification: The germanium element is classified under theMetalloid.
Phase at Room Temperature: This element is in a solid state at room temperature.
Density: The density of the germanium element is 5.323 grams per cm cubed.
Melting Point: The melting point of this element is 938°C, 1720°F.
Boiling Point: This element has 2833°C, 5131°F as its boiling point.
Discovered by: The germanium element was discovered by Clemens Winkler in the year 1886.
3. What are the applications of germanium that we use every day?
Germalloy, one of the most commonly used alloys contains this element. Other than that it is used in a wide range of electronics and semiconductor applications. Also, it is used as the optical material for fibre optics, lasers, and thermal management devices in computing as the germanium element is a very pure metal which has low density. Another more commonly used application of the germanium element is that it is used to make glass in many different colours.
4. How are germanium and silicon similar?
There are numerous similarities between silicon and germanium as the molecular weights (amounts of each atom) are the same. Germanium and silicon are both semiconductors. Germanium and silicon are both nice metals. Both materials have a similar colour- they're white or grey.
5. Are there any compounds of germanium found in nature?
Yes, there are some compounds of germanium which are found in nature. Some examples include Se-Ge alloy and GeAs crystals.









