




What Is Uranium? Key Facts Every Student Should Know
Uranium is a silvery-grey metal. It belongs to the actinide series of the periodic table. It occurs near the beginning of the actinide series. It has 92 protons and has 92 electrons out of which 6 are valence electrons.
Uranium
Uranium is weakly radioactive and it contributes to low levels of natural background radiation. Radium is always found with uranium ores because uranium is radioactive and always decaying. It plays an important role in the environment as it is used in nuclear power generation. Uranium is slightly paramagnetic.
Uranium Atomic Number and Symbol
Uranium is a chemical element which has the symbol U and has atomic number 92. Atomic mass of uranium is 238u.

Uranium Atomic Number
Who Discovered Uranium?
Uranium was discovered in 1789 by Martin Klaproth who is a German chemist. He isolated an oxide of uranium when he was analysing pitchblende samples. He named his discovery “Uran” after the planet Uranus which had been discovered eight years earlier.

The Discovery of Uranium Rays
Occurrence of Uranium
Uranium is found in many areas of the earth’s crust. Uranium deposits occur in association with igneous and sedimentary rocks. Uranium occurs naturally in low concentrations in soil, rock, and water and it is commercially extracted from uranium-bearing minerals such as uraninite. Uranium ore can be mined from open pits and underground excavations. This ore then can be crushed and treated at a mill to separate the metal uranium from the ore.
Uranium may be dissolved directly from the ore deposits in the ground. Uranium which is mined from the earth is stored, handled, and sold as uranium oxide concentrate.

Uranium Ore
Properties of Uranium
Uranium is a hard and heavy metal, silvery-white in colour. It is very dense, being 1.6 times more than lead.
It is solid at room temperature.
It is chemically toxic and its toxicity is more dangerous than its radioactivity.
It is reasonably soluble in water.
It has no taste and no smell.
Its powder is flammable and a fire hazard.
In the presence of an ultraviolet or black light, uranium makes the glass glow bright green.
In air, it tarnishes and when finely divided, it breaks into flames.
Uses of Uranium
Uranium is used in nuclear weapons.
It is also used in nuclear power plants.
Radium is always found in uranium ores because uranium is radioactive.
It is nowadays used to power commercial nuclear reactors that produce electricity and also to produce isotopes used for medical, industrial, and defence purposes around the world.
Uranium Periodic Table
Uranium is a chemical element on the periodic table. It is located in the seventh period in the periodic table. While it is easy to find many elements in the periodic table, uranium is placed below the main body of the periodic table in the actinide series which are transition metals.
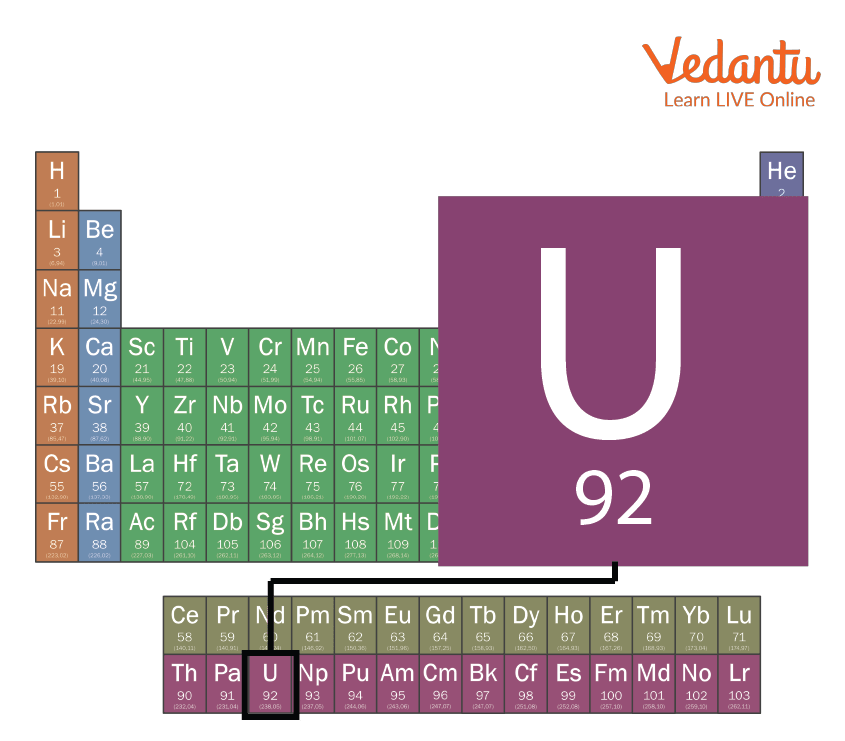
Uranium in the Periodic Table
Solved Questions
1. What happens to uranium after use?
Ans: Uranium undergoes radioactive decay very slowly after use.
2. How long will uranium stay in the body?
Ans: Uranium can stay in the bones for a long time. The half-life of uranium is 70-200 days, this is the time that uranium takes for half of it to leave the bones. Most of it which is not in bones leaves the body in 1-2 weeks.
3. Is uranium a hard metal?
Ans: Uranium is a dense, hard, and heavy metal having the highest atomic mass.
Summary
Uranium is a heavy metal which has been used as an abundant source of energy for over 60 years. It is important as it provides us with nuclear fuel that is used to generate electricity. It occurs in most rocks and in earth’s crust in the form of tin, tungsten, and molybdenum. It occurs in seawater and it can be recovered from oceans. Uranium occurs in three different forms known as isotopes. It has atomic number 92 and it has the highest atomic mass, that is, 238u. It is chemically toxic. It is radioactive.
FAQs on Uranium: Meaning, Properties & Everyday Uses
1. What is uranium and what are its key physical properties?
Uranium (symbol U, atomic number 92) is a very heavy, silvery-white metal that is weakly radioactive. It is a naturally occurring element found in low concentrations in soil, rock, and water. At room temperature, uranium is a solid and is one of the densest of all naturally occurring elements, even denser than lead. Its key property is its nuclear instability, which makes it useful for nuclear applications.
2. What are the main uses of uranium in today's world?
The primary application of uranium is as a fuel for nuclear power plants to generate electricity. Other significant uses include:
- Producing medical isotopes for diagnosing and treating diseases like cancer.
- Powering nuclear submarines and aircraft carriers in the military.
- Used in the production of nuclear weapons.
- Small amounts of depleted uranium are used as counterweights in aircraft and as radiation shields.
3. Is it safe to be near or touch uranium?
Uranium is both a toxic heavy metal and a radioactive substance, making it hazardous. While touching natural, unenriched uranium for a short period is not immediately lethal, it is strongly advised against. The main dangers come from:
- Chemical Toxicity: If ingested or inhaled, uranium can cause severe kidney damage.
- Radiation Exposure: Prolonged exposure, especially to enriched uranium, increases the risk of cancers, particularly lung cancer from inhaling uranium dust.
4. What is the difference between Uranium-235 and Uranium-238?
Uranium-235 (U-235) and Uranium-238 (U-238) are the two most common isotopes of uranium. The key difference lies in their nuclear properties:
- Abundance: U-238 is much more common, making up over 99% of natural uranium, while U-235 accounts for only about 0.7%.
- Fissile Property: U-235 is 'fissile,' meaning its nucleus can be easily split when hit by a slow-moving neutron, releasing a tremendous amount of energy. This makes it the primary fuel for nuclear reactors and weapons.
- Stability: U-238 is not fissile but is 'fertile.' It can absorb a neutron and eventually decay into Plutonium-239, which is fissile.
5. Why is uranium considered such a powerful energy source?
Uranium's power comes from the process of nuclear fission. The nucleus of a U-235 atom is highly unstable. When it absorbs a neutron, it splits into smaller atoms, releasing an immense amount of energy in the form of heat and radiation, along with more neutrons. These new neutrons can then trigger fission in other U-235 atoms, creating a self-sustaining chain reaction. The energy released from a tiny amount of uranium is millions of times greater than that from the same amount of coal or oil.
6. What is 'enriched uranium' and why is it necessary?
Enriched uranium is uranium in which the percentage of the U-235 isotope has been increased above its natural level of 0.7%. Enrichment is necessary because most nuclear power reactors require a higher concentration of U-235 (typically 3% to 5%) to sustain an effective nuclear chain reaction. The process involves separating U-235 from U-238, which is technically challenging because they are chemically identical and have very little difference in mass. The remaining uranium, with a lower concentration of U-235, is called depleted uranium.
7. How does a nuclear power plant use uranium to generate electricity?
In a nuclear power plant, uranium is used to generate electricity through a controlled process:
- Fission: Pellets of enriched uranium are placed in fuel rods inside a reactor. A controlled chain reaction is initiated, causing the uranium atoms to undergo fission and release a massive amount of heat.
- Heat Transfer: This heat is used to boil water, creating high-pressure steam.
- Turbine Generation: The steam is directed to a turbine, causing it to spin at high speed.
- Electricity Production: The spinning turbine is connected to a generator, which converts the mechanical energy into electrical energy, which is then sent to the power grid.
8. How much more energy does uranium produce compared to coal?
Uranium is incredibly energy-dense compared to fossil fuels like coal. As a practical example, one kilogram of Uranium-235 can produce about 24,000,000 kilowatt-hours (kWh) of energy. In contrast, one kilogram of coal produces only about 8 kWh of energy. This means that U-235 contains two to three million times more energy per unit mass than coal, making it a very efficient, though complex, source of power.









