




Who is Robert Boyle?
Robert Boyle was born in Ireland on 25 January 1627, he was a Great Anglo-Irish Philosopher. His work was well marked during the 17th century. He particularly belongs to the chemistry field but apart from chemistry, he works in the field of hydrostatics, medicine, physics, and earth sciences. His family was one of the richest in Britain. He is well renowned for his work in modern chemistry. He had a major contribution in the foundation of the royal society.
Robert Boyle
Early Years of Life
His family was considered as one of the richest families in Britain. At the tender age of eight years, he began his education at Eton college. Brother Frankies went on a great tour of the continent in 1639, his brother returned home while he pursued further studies in Geneva. In 1644, he returned to England, where he started his career in writing ethical and emotional tracks.
From 1647 to 1650, he remained in touch with natural philosophers and social reformers. Robert was very influenced by Galileo and he decided to promote the idea of Galileo that earth and other planets revolve around the Sun.
Robert Boyle's Inventions and Discoveries
He is generally famous for Boyle's law. Boyle's law states that at constant temperature, the volume of gas is inversely related with the pressure exerted on it.
He also gave explanations to metal calcination and combustion.
He put emphasis on chemical analysis and invented methods to measure purity and identify chemicals.
You also discover a vacuum pump and after conducting experiments show that sound could not be transmitted in vacuum and candle could not burn.
He shows that mathematical laws were followed by air.
Robert Boyle with his Vacuum Pump
Robert Boyle's Contribution to Chemistry
He is considered as the father of modern chemistry. He criticised Aristotelian and Paracelsian theories of philosophy. Boyle was able to chemically sublimate various substances from solid state to gas and vice versa without going through the liquid state. He tried to create a publicly accessible database of chemical analysis of every substance known at that time.
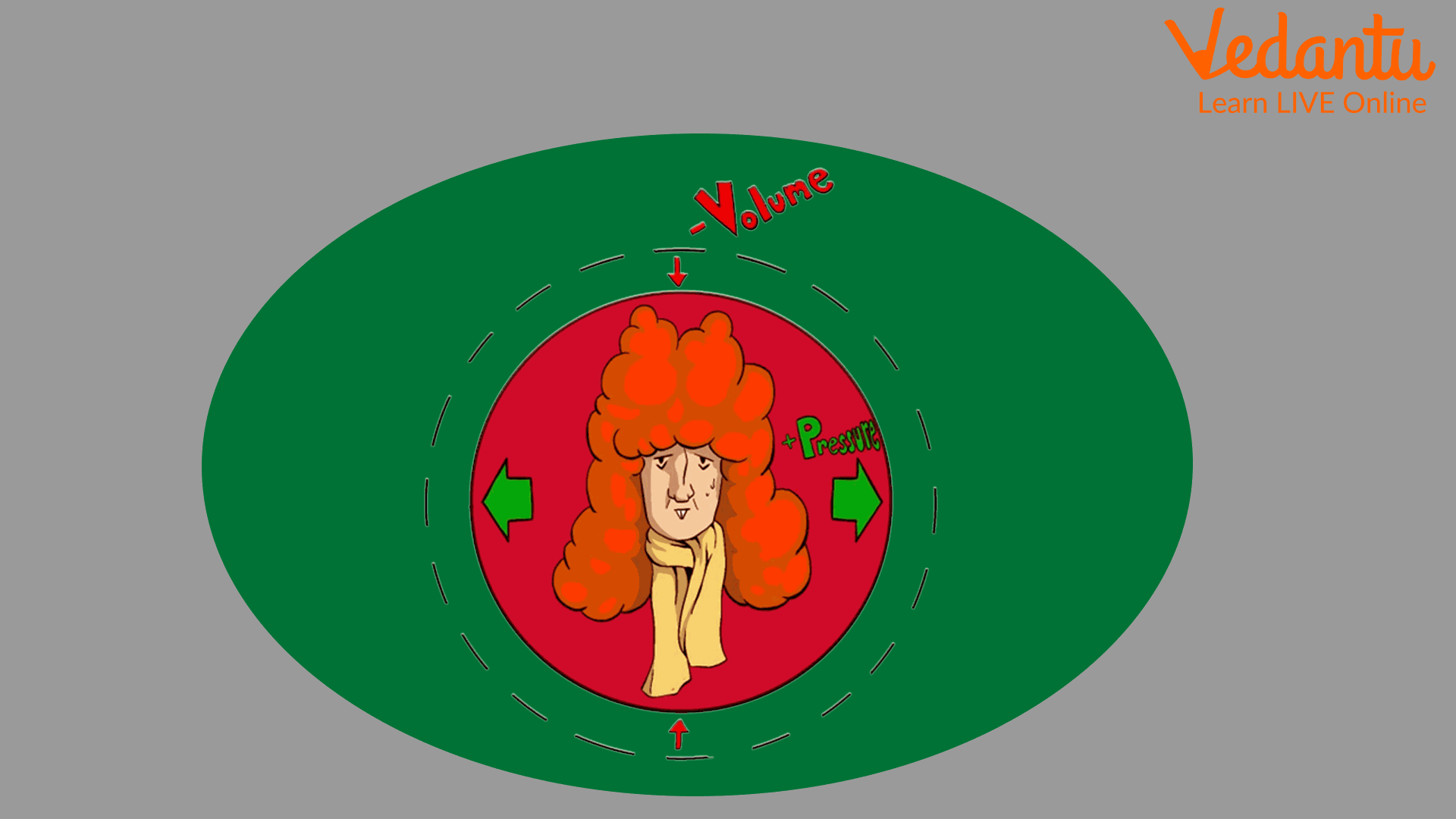
Boyle Law
Interest in Theology
He devoted a remarkable period of his time to theology. He strongly believes that the existence of God can be provided by the powerful evidence from natural philosophy. He also criticised some philosophers who denied to reveal much about God by the study of nature. In his lifelong career, he tried to show scientific support to Christianity.
Medicine
He was not a student of medicine but he had a deep interest in medicine. He never trusted the physician because when he was in Eton, a physician prescribed him wrong medicine due to which he became severely ill. He favoured mechanical theories and rejected Galen-based theories. He strongly believes that chemical remedies work better than Galenist. Oxford university gave him a doctorate of medicine degree Because of his respectable work in medicine.
Honours
Name of the law called Boyle’s law was given in his honour.
Robert Boyle prize for analytical science issued by Royal Society of Chemistry.
To honour the heritage of Robert Boyle Waterford Institute organises Robert Boyle summer school.
Solved Questions
1. Which society is founded by Robert Boyle?
Ans: Boyle was one of the Royal Society's original fellows. Through this Society, he disseminated his findings regarding the physical characteristics of air. He worked to develop chemistry as a mechanistic theory of matter-based mathematical science.
2. In Boyle's law, what quantity is constant?
Ans: Boyle noted that the relationship between pressure and volume is seen to be almost constant. For a perfect gas, the result of pressure and volume is a precise constant.
Summary
Apart from chemistry, he is interested in medicine, theology, Philosophy, and alchemy. He provides powerful evidence about God by study of nature; he was the founder of the Royal Society in the name given to Boyle's law in his honour.
FAQs on Robert Boyle Discovery
1. What is Robert Boyle's most famous discovery?
Robert Boyle's most famous discovery is Boyle's Law, which he formulated in 1662. This fundamental gas law states that for a fixed amount of an ideal gas kept at a constant temperature, the pressure and volume are inversely proportional. In other words, as the pressure on the gas increases, its volume decreases proportionally.
2. How does Boyle's Law work in real life? Explain with an example.
Boyle's Law is demonstrated in many everyday situations. A common example is a bicycle pump. When you push the handle down, you decrease the volume inside the pump's cylinder. This forces the air molecules closer together, increasing their pressure. This high-pressure air is then forced into the tyre. Another example is a medical syringe; pulling the plunger back increases the volume and decreases the pressure, drawing liquid in.
3. Why is Robert Boyle often called the “Father of Modern Chemistry”?
Robert Boyle earned the title “Father of Modern Chemistry” because he shifted the field from mystical alchemy to a rigorous, experimental science. His key contributions include:
- Emphasis on Experimentation: He championed the use of the scientific method, insisting that claims must be backed by empirical and repeatable experiments.
- Boyle's Law: He established a mathematical relationship for the behaviour of gases, a major step in quantitative analysis.
- The Sceptical Chymist: In his influential book, he challenged traditional theories like Aristotle's four elements and introduced the idea of elements as simple, indecomposable substances, laying the groundwork for modern element theory.
4. What is the mathematical formula for Boyle's Law as per the 2025-26 CBSE syllabus?
The mathematical representation of Boyle's Law is central to the study of the gaseous state. It can be expressed in two main ways:
- As a proportionality: P ∝ 1/V (Pressure is inversely proportional to Volume).
- As an equation: PV = k, where P is pressure, V is volume, and k is a constant value for a fixed mass of gas at a constant temperature.
For solving problems, the law is most often written as P₁V₁ = P₂V₂, where (P₁, V₁) are the initial pressure and volume, and (P₂, V₂) are the final values.
5. Did Robert Boyle discover the element oxygen?
No, this is a common misconception. While Robert Boyle conducted groundbreaking experiments on air, proving that it was necessary for combustion, respiration, and the transmission of sound, he did not discover oxygen as a distinct element. He worked with air as a whole. The credit for the discovery and isolation of oxygen later went to scientists like Joseph Priestley and Antoine Lavoisier, who built upon the experimental foundations Boyle had established.
6. What were Robert Boyle's other significant scientific contributions besides his famous gas law?
Beyond Boyle's Law, his scientific work was vast. He made several other important contributions:
- He conducted experiments on the nature of the vacuum using an improved air pump.
- He was among the first to study electricity, discovering that electric force could be transmitted through a vacuum.
- He published detailed definitions for chemical elements, compounds, and reactions that were far more precise than those used by alchemists.
- He also did pioneering work in the fields of physics, medicine, and philosophy, promoting a mechanical view of the universe.
7. How did Boyle's work fundamentally change the scientific approach to chemistry?
Robert Boyle's greatest impact was methodological. Before him, chemistry was largely dominated by the philosophical and often secretive practices of alchemy. Boyle advocated for a new approach based on openness, skepticism, and quantitative measurement. By insisting that experiments be recorded in detail and reproduced, he transformed chemistry into a legitimate, evidence-based scientific discipline, paving the way for the discoveries of Lavoisier and Dalton.
8. Are there any limitations to Boyle's Law?
Yes, Boyle's Law is not universally applicable. It perfectly describes the behaviour of an “ideal gas”, a theoretical concept. However, real gases deviate from this law under certain conditions, particularly at very high pressures or very low temperatures. This is because the law assumes gas particles have no volume and no intermolecular forces, which is not true for real gases. At extremes of temperature and pressure, these factors become significant and cause deviations from the predicted behaviour.









