




What Makes Atoms So Fascinating? Core Facts Explained for Young Learners
Look around you, any object no matter how different they are have one thing in common. Water has something in common with the bottle that it is stored in, the TV remote has something in common with the TV itself and the air has something in common with the cupboard.
If you are probably wondering what makes these various things related to one other, it’s the atoms. Atoms are simply the building blocks of any object. If you take any object and keep breaking them into smaller particles, at the end of the process, you are left with science atoms.
Atoms
How is an Atom defined?
An atom is defined as the basic building block of matter in the universe. So that’s one thing in common, but what makes matters different from one another?
It is interesting to note that though they all have atoms, atoms themselves are of different types. Different atoms make up different objects. As long as we are not looking into the atoms in core detail, these particles are pretty easy to understand and study.
For a long time science atoms were believed to be particles that could not be further divided into smaller species. After a point in time, once that hypothesis was disproved, subatomic particles were studied more in detail.
What are subatomic particles? These are smaller particles that are obtained when atom logos are divided further.
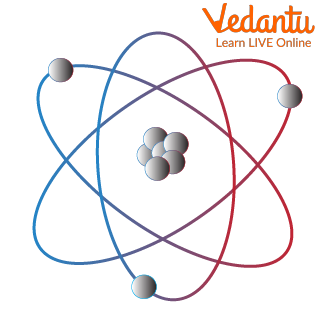
Basic Structure of an Atom
What are Subatomic Particles?
Subatomic particles are smaller in size when compared to science atoms. These are of 3 types on a much simpler level. These are electrons (negatively charged particles), protons (positively charged particles ), and neutrons (neutrally charged particles )
Electrons are almost weightless (their weights are so low and thus neglected) and revolve in orbits around the nucleus.
This can be visualised as the revolution of planets (or electrons in our case) around the sun (or the nucleus).
Protons have appreciable weight and are present in the centrally placed nucleus. They do not move around and are held in place within the nucleus. In accordance with our solar system analogy, they form the sun of the nucleus
Neutrons have appreciable weight but no charge. Their main function is to hold the protons together in the nucleus. Imagine neutrons to be the rope used in tug of war between two repelling sides.
Let us try to understand the atomic structure with an atom logo.
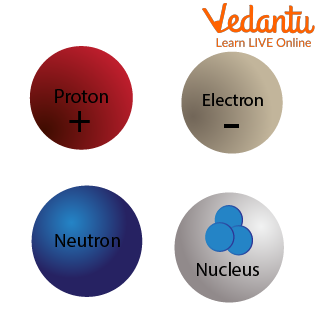
Subatomic Particles
Interesting Facts about the Atom
Let’s look into a few atom facts.
Atoms have a lot of space in them. The nucleus is so closely packed almost occupying very less to no space. To understand this better, if an atom is the size of a cricket stadium, the nucleus is the size of the cricket ball. The remaining space is just empty.
The word “atom” has a Greek origin, meaning “indivisible” or “uncuttable”. For a long time atoms were believed to be indivisible. With further experiments, they realised that atoms can be divided further into subatomic species.
Your body is made up of billions of atoms. About 99% of which are replaced every year producing new ones
Summary
Atoms are the basic building blocks of everything in the universe. All objects are made up of millions of atoms. Revisiting atom facts, atoms were believed to be indivisible particles and hence got their name. With further advancements in sciences, scientists realised atoms can be further divided producing sub-atomic particles.
Subatomic particles include electrons, protons, and neutrons. Electrons and protons are charged species that are equal in number in a neutral atom. Neutrons are neutrally charged and help hold the protons within the nucleus. The nucleus of an atom is centrally placed around which electrons revolve along an orbit as planets revolve around the sun.
FAQs on Interesting Facts About Atoms for Kids
1. What is an atom in simple words for kids?
An atom is the smallest, most basic building block of everything in the universe. Imagine everything you see—your chair, the water you drink, and even the air you breathe—is built from tiny, invisible LEGO bricks. Atoms are those bricks. They join together to make up all solids, liquids, and gases.
2. What are the main parts of an atom?
An atom is made of three tiny particles: protons, neutrons, and electrons. The protons (positive charge) and neutrons (no charge) are packed together in the center, called the nucleus. The electrons (negative charge) are much smaller and buzz around the nucleus at super high speeds, almost like tiny planets orbiting a sun.
3. Can you share some interesting facts about atoms?
Certainly! Here are some fun and interesting facts about atoms:
They are mostly empty space: Over 99.9% of an atom's volume is just empty space between the nucleus and the electrons.
You are made of trillions of atoms: The average human body contains about 7 octillion atoms (that's a 7 followed by 27 zeros!).
Atoms are ancient: The atoms that make you up have been around for billions of years, existing in different forms before becoming part of you.
The name means 'uncuttable': The word “atom” comes from the ancient Greek word 'atomos,' which means indivisible or uncuttable, because they once thought it was the smallest possible thing.
4. If atoms are over 99% empty space, why don't we fall through the floor?
This is a great question! Even though atoms are mostly empty, we can't pass through solid objects because of the electrons. The electrons in the atoms of the floor repel the electrons in the atoms of your feet. This powerful pushing force between the electron clouds is what makes objects feel solid and prevents you from falling through.
5. How can the same few types of particles build everything from a person to a rock?
It's all about the arrangement and the numbers. The number of protons in an atom's nucleus determines what element it is. For example, an atom with one proton is always hydrogen, while one with eight protons is always oxygen. These different elements then act like a set of ingredients that can be combined in countless ways to form molecules—the building blocks of everything we know, from water (H₂O) to sugar.
6. Why can't we see a single atom, even with a normal microscope?
We can't see atoms with our eyes or a regular light microscope because they are incredibly small—far smaller than the wavelength of visible light. Light waves essentially pass right over them without bouncing back to our eyes, making them invisible. It's like trying to see a single grain of sand by throwing a beach ball at it; the ball is too big to detect it. Scientists need special, powerful tools like electron microscopes to get an image of individual atoms.
7. Do atoms ever actually touch each other?
In the way we think of touching, no. The solid part of an atom (the nucleus) never actually touches the nucleus of another atom in everyday situations. What we feel as 'touch' is the force of the negatively charged electron clouds of atoms pushing against each other. It's similar to how two magnets with the same poles facing each other will push apart without physically touching.
8. What happens when an atom loses or gains an electron?
When an atom loses or gains an electron, it becomes electrically charged and is called an ion. Normally, an atom has an equal number of protons (positive) and electrons (negative), making it neutral. If it loses an electron, it has more positive charges and becomes a positive ion. If it gains an electron, it has more negative charges and becomes a negative ion. This is the basic idea behind static electricity!









