




Atomic Structure Explained: Protons, Neutrons & Electrons
The atom is the fundamental structure for all matter known to humans. Atoms are tiny, and atoms are made up of a few much smaller particles. The fundamental particles that make up an atom are electrons, protons, and neutrons. Atoms fit along with different atoms to make up matter.
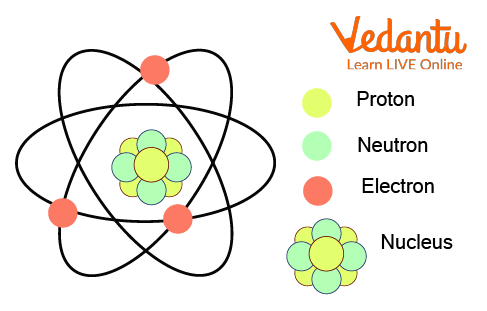
What is an Atom
It takes a large number of atoms to make up anything. There are too many atoms in the human body; we cannot count the number. There are various types of atoms depending upon the number of electrons, protons and neutrons every atom contains. Each unique type of atom makes up an element. There are 92 natural elements and up to 118 when you include man-made elements.
Inside the Atom
The Proton
The proton is a positively charged particle situated at the centre of the atom, inside the nucleus of an atom. The hydrogen atom is unique because it just has a single proton and no neutron in its nucleus.
The Electron
The electron is a negatively charged particle that spins around the nucleus. Electrons spin so quickly around the nucleus that researchers can never be 100 percent sure where they are found, yet researchers can mathematically assume where electrons should be. If there is a similar number of electrons and protons in an atom, then the atom is said to have a neutral charge. Electrons are drawn to the nucleus by the positive charge of the protons. Electrons are multiple times smaller than neutrons and protons.
The Neutron
The neutron has no charge. They are present inside the nucleus of an atom, along with the protons. The number of neutrons influences the mass and the radioactivity of the atom.
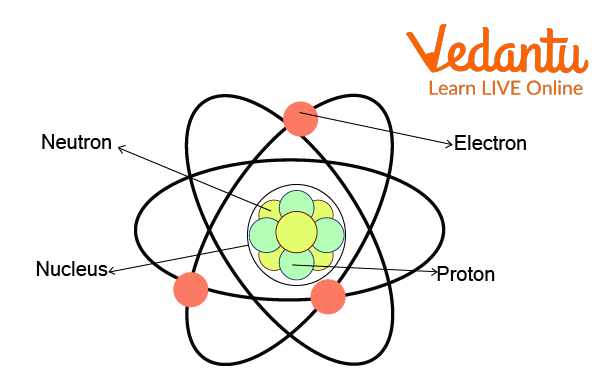
Components of an Atom
Subatomic Particles of an Atom
For a long time, it was accepted that atoms are particles that matter is composed of and that these atoms can't be divided further. The observations of experiments related to an atom directed during the last half of the 19th century and early 20th century proved that the atom isn't a definitive particle. Scientists further confirmed the discovery of subatomic particles. The three fundamental subatomic particles that comprise an atom are electrons, protons, and neutrons.
Summary
Atoms have a very long life; they can stay forever also. They can change through chemical reactions, sharing electrons with different atoms. However, the nucleus is extremely difficult to part, meaning most atoms are around for quite a while.
Protons and neutrons are present in the atom's nucleus, whereas electrons revolve around them. The number of protons present in the nucleus of an atom decides which particle is going to produce. For example, if there are 79 protons in the nucleus of the atom, then it makes gold. If there are 8 protons in the nucleus of the atom, then it makes oxygen gas. In the same manner, many other elements are formed on our planet.
FAQs on Inside the Atom: Structure and Key Components
1. How much space is in an atom?
Hydrogen atoms are about 99.9999999999996% empty space. Put another way, if a hydrogen atom were the size of the earth, the proton at its centre would be about 200 metres (600 feet) across.
2. Are all matter made of atoms?
Everything in the universe (except energy) is made of matter, and so everything in the universe is made of atoms. An atom itself comprises three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons.
3. Can your hand go through atoms?
Atoms are extremely small particles that are only visible through microscopes. Thus, it is impossible to say that a ‘hand go through atoms.’









