




Why Understanding the History of Platinum Matters for Students
Could you name a precious silver and white metal which is used to make jewellery? Maybe your mum has a locket or a bracelet made from it. Let us give you one more hint. It is a type of metal which has good resistance to chemical attack and corrosion.
There are various metals which are used to make jewelleries, perhaps the one which is silver and has high resistance to chemical reactions is platinum. Platinum got its name from the Spanish word Platina which means little silver. Gold is the element that comes after Platinum. Let’s now see about its history, where platinum is commonly found and much more.

Appearance of Platinum
What Is Platinum?
Platinum is a silver and grey colour metal which is hard but stylish. It is found on the 10th column of the third row of the periodic table. Platinum has its chemical symbol as "Pt". The metal is transitioned and has an atomic number of 78. It has 78 protons and electrons and several 117 neutrons. Platinum has an atomic weight as 195.08
Palladium is an element which comes after platinum. The platinum group consists of various elements such as platinum, palladium, rhodium, ruthenium, iridium, and osmium. All these elements consist of similar properties and they occur together in nature.
History of Platinum
The only place where platinum was found earlier in the 1800s was in Colombia. After a few years, the existence of platinum was found in Russia. Later on, the one country where the metal was found in abundance to an extent that it became the largest supplier of it was Canada.
People started utilising platinum for making catalysts which increase the reaction of certain chemicals. After a quarter of a century, platinum was discovered in South Africa which supplies 75% of Platinum to the world and it is now the largest supplier of platinum.
Where Is Platinum Found?
The existence of platinum is very few in the world. A majority of 80% of platinum is found in South Africa and the second largest place is Russia, which is 10 percent and the rest is found in North and South America. Geographically, platinum is found underground, mostly in nuggets or grains and is usually plated with other metals, such as gold, nickel and copper ores or mineral sperrylite. It is rare to find platinum on its own.

Mining of Platinum in South Africa
Where is Platinum Found in Nature?
Platinum is the most precious metal that has been ever discovered. These are only 0.005 parts per million present on the planet, in comparison with the other metals like gold and silver
Where is Platinum Found in the World?
It is present in a few numbers on the earth. Research says that the availability of it is more on the moon or the metroids. Platinum is primarily found in compounds with other metals in the platinum-like group on the earth and must go through a mining and purifying process in order to be found and distributed for use.
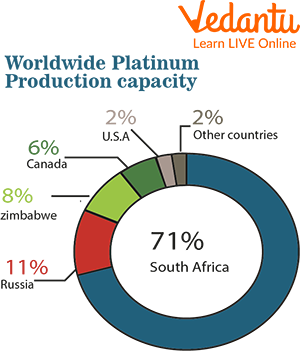
Reserves of Platinum in Various Countries
Characteristics and Properties of Platinum
The characteristics of platinum are as follows:
The major characteristics of platinum include silver and greyish colour and extremely shiny. It is very ductile which means it is easily bendable and can be stretched and drawn into wire without breaking it.
Platinum has a high resistance to corrosion unlike most other metals when it contacts air it does not tarnish or rust. It is also very dense (one of the highest of the elements) and has a high melting point. Platinum has a high melting point of approximately a thousand degrees centigrade.
Though platinum has high chemical resistance, it can be dissolved in the combination of extremely high acidic chemical of nitric and hydrochloric also known as aqua regia
Platinum is extremely stable which means it is not reactive in the environment and it keeps its properties and usefulness intact. It is also known as a noble metal with these characteristics.
Uses of Platinum
Some uses of platinum are as follows:
Jewellery is frequently made of platinum. However, catalytic converters for vehicles, lorries, and coaches are where it is primarily used. Each year, this meets around 50% of the need. The conversion of engine emissions into less dangerous waste products is made possible by platinum.
In the chemical process industries, platinum is utilised as a catalyst in the manufacture of benzene, silicone, and nitric acid. To increase the effectiveness of fusion reactors, it is often used as a catalyst.
Platinum use is wide and more frequent in the electronic sector for the computer hard disks and thermocouples.
Additionally, gas turbines, LCD screens, spark plugs, cardioverters, and dental fillings are all made of platinum.
Important chemotherapeutic medications used to fight cancer include platinum compounds.
Summary
In the article, platinum for kids, we have discussed the history of platinum, where it is found, its characteristics and its uses. Platinum is also a chemical element, but it has some special properties that make it relatively hard to find in nature and expensive to extract. Platinum is mostly used as an electrical conductor because it doesn't corrode or chip easily when exposed to high temperatures or aggressive chemicals. Gold is the element that comes after platinum. We hope you have understood the concepts concerning the element platinum.
FAQs on History of Platinum: Discovery, Uses & Impact
1. What are the main industrial and commercial uses of platinum?
Platinum is a highly versatile metal with several important industrial and commercial applications. Its primary uses include:
- Catalytic Converters: The largest use of platinum is in vehicle exhaust systems, where it acts as a catalyst to convert toxic pollutants like carbon monoxide and nitrogen oxides into less harmful substances.
- Jewellery: Due to its rarity, durability, and natural white sheen that doesn't tarnish, platinum is a highly sought-after material for high-end jewellery.
- Electronics: It is used in manufacturing computer hard disks, thermocouples for temperature measurement, and in producing optical fibres and LCDs.
- Medical Field: Platinum is biocompatible and corrosion-resistant, making it ideal for medical implants like pacemakers. Certain platinum compounds are also crucial in creating chemotherapy drugs to treat cancers.
2. How is platinum used in everyday objects that we might not be aware of?
Beyond its famous use in jewellery, platinum is present in many everyday items. For example, it is used in the tips of high-performance spark plugs in cars for better longevity and efficiency. It is a key component in the production of silicone, which is used in everything from kitchen utensils to electronics. Additionally, it plays a role in manufacturing fertilisers and even in refining crude oil to produce high-octane gasoline.
3. Is platinum a magnetic material? Explain the science behind it.
In its pure form, platinum is not magnetic. It is a paramagnetic metal, which means it is very weakly attracted to a magnetic field but does not retain any magnetic properties once the field is removed. However, platinum can become magnetic when it is alloyed (mixed) with certain other metals, most notably cobalt. This platinum-cobalt alloy exhibits strong magnetic properties and is used in specific industrial applications.
4. What makes platinum a suitable material for fine jewellery?
Several unique properties make platinum an excellent choice for crafting fine jewellery. Firstly, it is naturally hypoallergenic, making it safe for people with sensitive skin. Secondly, it is extremely dense and durable, meaning it holds gemstones securely and resists wear and tear. Thirdly, platinum has a natural, radiant white lustre that does not fade or tarnish over time, so it doesn't require frequent replating like white gold does. Finally, it is one of the purest precious metals used in jewellery, typically at 95% purity.
5. Why is platinum so useful across many different industries?
Platinum's widespread utility stems from its remarkable physical and chemical properties. Its extremely high melting point (1,768°C) and resistance to corrosion and chemical attack make it incredibly durable for industrial processes. Its most significant property is its ability to act as a highly effective catalyst, speeding up chemical reactions without being consumed itself. This is vital in the automotive, chemical, and petroleum refining industries. Its biocompatibility and conductivity also make it indispensable in medical and electronic applications.
6. How does platinum actually work as a catalyst in a car's exhaust system?
Inside a catalytic converter, platinum is coated onto a ceramic honeycomb structure to maximise its surface area. As hot, toxic exhaust gases like carbon monoxide (CO) and nitrogen oxides (NOx) pass over this platinum-coated surface, the platinum provides an active site for chemical reactions to occur more easily. It helps oxidise the harmful CO into less harmful carbon dioxide (CO₂) and reduces the NOx into harmless nitrogen gas (N₂). Essentially, it provides the right environment for these chemical transformations to happen almost instantly, cleaning the exhaust before it leaves the tailpipe.
7. What are the key differences in the uses of platinum compared to palladium?
While both platinum and palladium are precious metals used as catalysts, their applications differ based on properties and cost.
- Primary Use: Platinum is more effective at catalysing reactions in diesel engines, while palladium is more commonly used and more cost-effective for petrol engines.
- Jewellery: Both are used in jewellery, but platinum is denser, heavier, and generally considered more premium. Palladium is lighter and was often a more affordable alternative.
- Density and Price: Platinum is significantly denser than palladium. Their prices fluctuate based on industrial demand, but historically, platinum has often been more expensive.
8. Is it true that platinum is rarer than gold?
Yes, it is true. Platinum is significantly rarer than gold. It is estimated that all the platinum ever mined would fit into a small room, whereas gold is mined in much larger quantities annually. This rarity is a major factor contributing to its high value. Furthermore, platinum is often found deeper in the earth and is more difficult and costly to mine and refine than gold, which also impacts its market price and perception as a premium metal.









