




Introduction
All forms of matter contain heat energy. No matter if it is a volcano or an ice cube, all have some amount of heat. This heat energy can also be transferred from one substance to another; for example, when ice is melting, the heat energy from the ice cube is transferred to the surroundings, and thus the ice melts, or in the case of a volcano, the heat energy stored inside it in the form of lava is also transferred to its nearby surroundings when the volcano erupts. There is always a transfer of energy between substances.
There are three ways of transferring heat energy. The first is conduction, which occurs in fluids at rest, such as this metal bar. The second one is convection, in which molecules of fluid and air transfer heat. And the third form of heat transfer is radiation, which occurs without a material carrier. Here are only some of your choices for heating energy sources: natural gas, propane (LP), oil, coal, wood, electricity, heat pumps, ground source heat pumps and solar energy. Heat is measured in Celsius, Kelvin, or Fahrenheit.
Heat Energy
Heat Energy for Kids
Heat is defined as the flow of energy from an object at higher temperature to the object which is at lower temperature.In our day to day terms we refers heat to something which basically have higher temperature or warmer,but in real term heat is the flow of molecules from higher temperature region to lower temperature region due to this flow, the cooler part becomes hot because hot molecules transfer their heat energy to cold molecules.

Fire Produces Heat
Facts About Heat
Two objects having less temperature can Create heat when they are rubbed together because the movement of molecules can create heat, so when we rub objects, molecules start moving, producing heat.
When we use the air conditioner during hot days, these days seem hotter than actually they are. The same is the case for cold days when we use the heater during cold days, they seem colder than they actually are. So, due to use of these appliances our tolerance power decreases.
Warm objects expand or become larger.
Heat transfers from one object to another until both objects are at the same temperature.
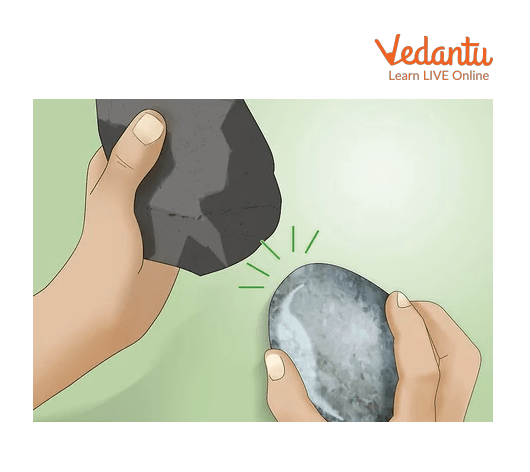
Showing Objects Rub Together
Heat Information
Let’s have two bodies at different temperatures. Let the temperature of the first body is 100 degree Celsius and the temperature of another body is 40 degree Celsius. When these two bodies are brought together, the temperature of the body which is at 100 degree Celsius, will transfer to the body which is at 40 degree Celsius. This transfer of heat continues until both bodies attain equal temperatures. In simple words, when the temperature of both bodies becomes 70 degree Celsius they stop the transfer of heat.
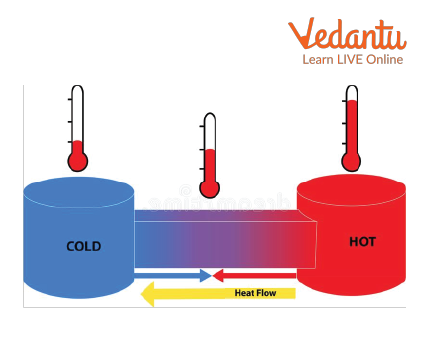
Transfer of Heat From Hot object to Cold Object
Heat Science
In scientific terms, the study of heat is called thermodynamics. Above in this article, we already read that heat energy is produced due to the movement of molecules, and in terms of science, the energy produced by the movement of molecules is called kinetic energy. So, both heat energy and kinetic energy are related to each other hence we can say that heat in any object is due to the kinetic energy of the molecules of the object.
Heat Transfer
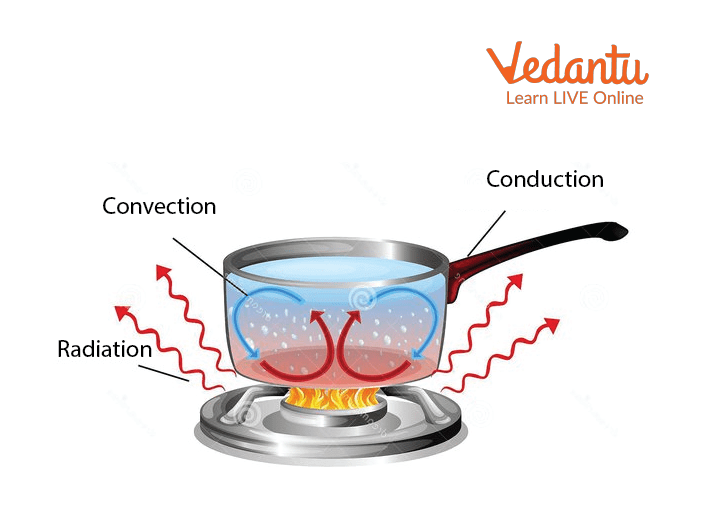
Conduction,Convection and Radiation
Transfer of heat can be through conduction,convection and radiation. Let’s discuss each:
Conduction:
Conduction is the transfer of energy from one molecule to another by direct contact of objects eg: when one end of rod is heated and other end remains unheated then after a few minutes we find the temperature of the whole rod is the same,this is because of direct contact of molecules.

Conduction
Convection:
Convection is the form of heat transfer by the fluid, e.g. when water is heated, some molecules of water which are present at the bottom can be heated and molecules at the top remain unheated. So, these heated molecules transfer their heat energy to the unheated molecules.
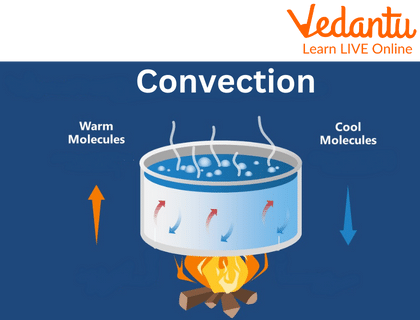
Convection
Radiation:
Radiation is the form of heat which is transmitted in the form of rays and waves, e.g.: heat from the sun which heats the earth's surface.
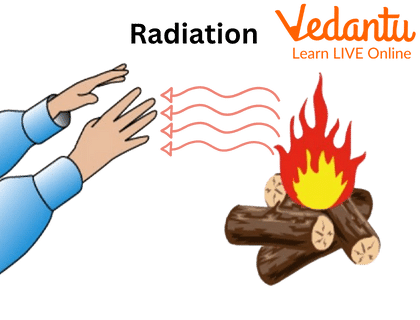
Radiation
Solved Examples
Q1. What is the biggest and the primary source of heat energy?
Ans. The sun is the biggest and the primary source of heat energy.
Q2. Why heat transfer?
Ans. Heat transfer due to the movement of molecules.
Summary
In this article we learned that all matter contains heat energy. Heat energy is the result of the movement of tiny particles called atoms, molecules or ions in solids, liquids and gases. Heat energy is defined as flow of energy from hot object to cold object. Heat can be transferred through different processes called convection, conduction, and radiation.
FAQs on Heat Energy for Kids
1. What is heat energy, explained in simple words for kids?
Heat is a form of energy that makes things feel warm or hot. It is created by the movement of tiny particles called atoms and molecules. The faster these particles move, the more heat energy is produced. You can feel heat energy from the sun, a fire, or even by rubbing your hands together quickly.
2. What are the most common sources of heat energy?
The main sources of heat energy that we encounter are:
- The Sun: The biggest and most important source of natural heat for our planet.
- Burning Fuels: Burning things like wood, coal, and gas releases stored chemical energy as heat. This is how we cook food and warm our homes.
- Electricity: Electrical appliances like heaters, toasters, and ovens are designed to convert electrical energy into heat energy.
- Friction: When two objects rub against each other, they create friction, which produces heat.
3. How does heat travel from a hot place to a cold place?
Heat energy moves in three different ways:
- Conduction: This happens in solids. Heat travels from one particle to the next, like a chain reaction. For example, when you heat one end of a metal spoon, the other end eventually gets hot.
- Convection: This occurs in liquids and gases. The warmer, lighter fluid rises, and the cooler, denser fluid sinks, creating a circular flow called a convection current. This is how water boils in a pot.
- Radiation: Heat travels in waves through empty space, without needing particles. This is how we feel the heat from the Sun or a campfire, even from a distance.
4. What is the difference between heat and temperature?
While they are related, heat and temperature are not the same. Heat is the total energy of all the moving particles in an object. Temperature is the measure of how hot or cold an object is, which tells us the average energy of the particles. An iceberg has more total heat energy than a cup of hot tea because it's much bigger, but the tea has a much higher temperature.
5. Why do we wear woollen clothes in winter but cotton clothes in summer?
This is because of how different materials handle heat. Wool is a poor conductor of heat, or an insulator. It traps a layer of air close to our body, and this trapped air prevents our body heat from escaping, keeping us warm. Cotton, on the other hand, allows air to circulate easily and absorbs sweat, which then evaporates and cools our skin, making it ideal for summer.
6. What is the difference between a heat conductor and an insulator?
A conductor is a material that allows heat to pass through it easily and quickly. Metals like steel, copper, and aluminium are good conductors, which is why cooking pans are made of them. An insulator is a material that does not allow heat to pass through it easily. Wood, plastic, glass, and air are good insulators, which is why pot handles are often made of plastic or wood.
7. What are some examples of using heat energy in our daily lives?
We use heat energy every day for many important tasks. Some common examples include:
- Cooking food on a stove or in an oven.
- Boiling water to make tea or coffee.
- Using a geyser or water heater for a warm bath.
- Ironing clothes to remove wrinkles.
- Drying wet clothes in the sun.
- Warming our homes with a room heater.
8. If you touch a metal chair and a wooden chair in the same room, why does the metal one feel colder?
This is a great question about how heat transfer works! Both chairs are actually at the same room temperature. However, metal is an excellent conductor of heat. When you touch it, it quickly draws heat away from your hand, making your hand feel cold. Wood is an insulator, so it transfers heat much more slowly. Since it doesn't pull heat from your hand as fast, it doesn't feel as cold to the touch.





















