




What Is Radioactivity? Key Principles, Benefits, and Everyday Uses Explained
Radioactivity is the ability of some substances to release energy and subatomic particles spontaneously. In essence, it is a characteristic of particular atomic nuclei. An unstable nucleus will spontaneously decay or change into a more stable structure, but this will only happen in a handful of specific ways by ejecting particular particles or electromagnetic radiation. Numerous naturally occurring elements and their synthetic isotopes exhibit the property of radioactive decay. We will discuss radioactivity in this article. This process is a very important topic in chemistry. So let’s make chemistry easier!

Radioactivity
Radioactivity Definition Chemistry
Radioactivity is the property that some materials have to emit energy into the environment. It usually appears in the form of electromagnetic radiation that is cast in the form of subatomic particles. Depending on where you are in the electromagnetic field, this could be high or low-frequency radiation. It is a phenomenon that occurs due to nuclear energy instability in the atomic nucleus.

Cause of Radioactivity
Different Types of Radioactivity: Alpha, Gamma, and Beta Radiations
The three most common types of radiation are gamma rays, beta particles, and alpha particles.
Alpha Radiation
It is a particle that releases an unstable nucleus. Two protons and two neutrons make them up. Therefore, alpha radiation is considered a completely naked ice atom without any electrons. The alpha particle carries a positive charge since an atom's nucleus contains two protons. Alpha radiation is very little penetrating and is easily stopped by a sheet of paper. This is usually a very short range in the air. Uranium-238 and radium-226 are two atoms that release alpha radiation as examples.
Beta Radiation
This radiation is ionising and occurs in about a meter of air. This can be prevented by a sheet of aluminium foil. A positron releases an electron as radioactive decay progresses. Both are of nuclear origin. That is why there are two subtypes of beta radiation: beta positive and beta negative. The first is due to the emission of an electron of nuclear origin with a positive charge, and the second is due to the emission of an electron of nuclear origin and a neutron which turns into a proton.
Gamma Radiation
It is radiation of an electromagnetic nature. It is a powerful and penetrating wave that is stopped only by the lead. This penetration ability allows its use in the form of cobalt-60 in the treatment of cancer in deep sites in the body.
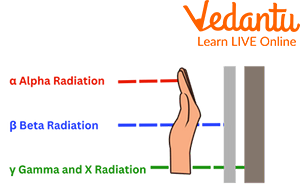
Types of Radiation
Causes of Radioactivity
Radioactivity is the phenomenon of spontaneous emission of particles or waves from unstable nuclei of some elements. Alpha, beta, and gamma are the three different forms of radioactive emissions. Alpha particles are positively charged helium (He) atoms, beta particles are negatively charged electrons, and gamma rays are neutral electromagnetic radiation.
In nature, atoms can be either stable or unstable. If there is a balance in the forces acting on the constituents of the nucleus, an atom will be stable. An atom is unstable (radioactive) if these forces are unbalanced or if there is an excess of internal energy in the nucleus.
What is Radioactive Decay?
Ionising radiation is produced as a result of radioactive decay. Ionising radiation is a health risk because it can harm the atoms in living things, damaging tissue and the DNA in genes. The discharged ionising radiation may contain alpha particles. Atoms of a radioactive substance emit alpha (α) or beta-particles and gamma rays. This changes the atomic weight and the atomic number of the radioactive material. In this way, the initial radioactive atom decays and the atom of a new element is formed. This phenomenon is called radioactive disintegration or Radioactive decay.
In fact, radioactive decay is a nuclear process, i.e. the nucleus of the atom emits Radioactive rays. This process cannot be accelerated or slowed down by physical or chemical means.
A radioactive element's half-life, or the amount of time it takes for one half of any given quantity of the isotope to decay, is used to describe the rate of radioactive element decay. Half-lives for various nuclei range from more than 1024 years to fewer than 1023 seconds. The daughter of the parent isotope, which is the end result of a radioactive decay process, may be unstable, in which case it will also degrade. Up till a stable nuclide has been produced, the process goes on.
Radioactive Facts
Most things like cell phones produce radiation. As most of the radiation exposed daily is non-ionizing, it is generally not dangerous.
A particular type of fungus is found growing within the Chernobyl reactors that actually thrives on radiation.
Uranium samples were inside a toy that was released in the 1950s.
Radiation that occurs naturally is all around us. Our soils, air, and water all contain radiation, as do we. We come into contact with it every day through the food we eat, the water we drink, and the air we breathe since it happens in our natural environment.
Summary
To conclude all the conceptual understanding regarding radioactivity in this article, we can say many elements found in nature are unstable in nature. Some ionising radiation and energy are continuously emitted from the nucleus of such an atom. This process is called radioactivity. Elements that are not stable and whose nucleus emits radiation continuously are called radioactive substances. This was all about radioactivity. This article will help you understand one of the most important topics of Chemistry with ease.
FAQs on Radioactivity Facts Every Student Should Know
1. What is radioactivity?
Radioactivity is the natural phenomenon where the unstable nucleus of an atom spontaneously disintegrates, or decays, to form a more stable nucleus. During this process, the nucleus releases energy in the form of particles or electromagnetic waves, collectively known as ionising radiation.
2. What are the main types of radioactive decay?
The three most common types of radioactive decay are:
- Alpha (α) decay: The emission of an alpha particle, which is a helium nucleus (two protons and two neutrons).
- Beta (β) decay: The emission of a beta particle, which can be an electron (beta-minus) or a positron (beta-plus).
- Gamma (γ) decay: The emission of high-energy photons called gamma rays, which often occurs after alpha or beta decay to release excess energy from the nucleus.
3. Why do some atomic nuclei become radioactive?
An atomic nucleus becomes radioactive or unstable when the forces holding it together are imbalanced. This is typically caused by an unfavourable neutron-to-proton ratio. For a nucleus to be stable, the strong nuclear force must overcome the electrostatic repulsion between the positively charged protons. If there are too many or too few neutrons for the number of protons, the nucleus has excess energy and will undergo radioactive decay to reach a more stable, lower-energy state.
4. How are alpha, beta, and gamma radiation different from each other?
Alpha, beta, and gamma radiations differ primarily in their composition, charge, and penetrating power.
- Alpha (α) particles: Are positively charged helium nuclei. They have low penetrating power and can be stopped by a sheet of paper or human skin.
- Beta (β) particles: Are high-energy electrons or positrons. They are more penetrating than alpha particles and can be stopped by a thin sheet of aluminium.
- Gamma (γ) rays: Are high-energy electromagnetic radiation with no charge. They are the most penetrating and require thick lead or concrete to be blocked.
5. What are some common real-world applications of radioactivity?
Radioactivity has several important applications across various fields, including:
- Medicine: Used in diagnostics (e.g., PET scans) and cancer treatment (radiotherapy using Cobalt-60).
- Energy: Nuclear power plants use the decay of elements like uranium to generate electricity.
- Industry: For sterilising medical equipment and in gauges to measure the thickness of materials.
- Archaeology: Carbon-14 dating is a key example used to determine the age of ancient organic artefacts.
6. What are the primary dangers of exposure to radioactive materials?
The main danger of radioactivity is its ionising radiation, which carries enough energy to strip electrons from atoms in living cells, causing damage to tissue and DNA. High doses of radiation can lead to Acute Radiation Syndrome (ARS), while long-term exposure to lower doses significantly increases the risk of developing cancer later in life due to DNA mutations.
7. What is meant by the 'half-life' of a radioactive element?
The half-life (T₁/₂) of a radioactive element is a fundamental concept defining the time it takes for half of the radioactive nuclei in a sample to decay. This value is a constant for each specific isotope and is not affected by external physical or chemical conditions. For example, if an element has a half-life of 100 years, a 100g sample will be reduced to 50g after 100 years and to 25g after another 100 years.
8. Is radioactivity a natural phenomenon or is it always man-made?
Radioactivity is a completely natural phenomenon. Many elements found in the Earth's crust, such as uranium and thorium, are naturally radioactive. We are constantly exposed to low levels of natural background radiation from soil, rocks, the air we breathe (e.g., radon gas), and even from elements within our bodies like potassium-40. While some radioactive isotopes are produced artificially, the process itself is a natural property of unstable atoms.









