




What Makes Platinum Unique? Key Properties & Everyday Uses
Chemistry is one of the most important subjects of science that deals with different properties like bond formation, chemical equations, etc. The periodic table has several elements which are used in chemistry and even in our daily life. The arrangement of elements in the periodic table is based upon increasing atomic number. There are about 118 elements, among which, one is the element called platinum.

Picture of the Periodic Table
There are several interesting facts about platinum that one can discover through the placement of platinum on the periodic table like how many protons platinum has along with the physical and chemical properties of platinum. Let us read ahead to discover these facts about platinum.
Understanding Properties with Symbol of Platinum
The element platinum is the 3rd element located on the 10th column of the periodic table. According to its position on the periodic table, platinum is considered a transition metal that contains about 78 electrons, 78 protons, and 117 neutrons, making it one of the most abundant isotopes present in nature.
The symbol of platinum is very easy to remember since it contains only two letters, a capital P and a small letter t which are denoted together like ‘Pt’. The name platinum comes from the roots of the Spanish language where the form 'Platina’ means silver which is the appearance of Platinum.
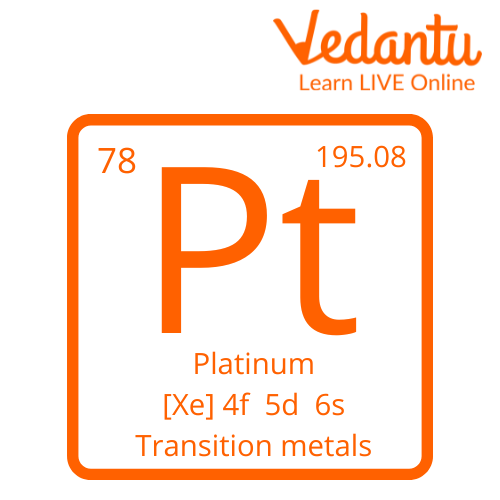
Symbol of Platinum
Some of the key properties everyone should remember about platinum are:
Atomic Number- 78
Atomic Weight- 195.084
Discovered by People of South America
Characteristics and Chemical Properties of Platinum
According to the characteristics of platinum, through its appearance, it looks like shiny, silvery metal and has physical properties such as ductility, which can make it drawn into thin wires. It is also malleable, which means platinum can be easily pounded to take different shapes.
According to the chemical properties of platinum, it does not start corroding when it comes into contact with air and is resistant to corrosion. Platinum is also considered one of the densest elements present in nature and has a very high melting point. Platinum is also considered to be one of the inactive elements but has exceptions such as it dissolves in aqua regia and hot alkalis.
How Is Platinum Used in Everyday Life?
Platinum uses in everyday life has many aspects. But since platinum is very rare to be found, it makes it expensive and ultimately is used by people for making investments as currencies. Apart from such usages, it is also considered a very beautiful metal for jewellery and artworks due to its properties of ductility and malleability.
It is also used as a catalyst in the automobile and the petroleum industry since it helps in driving different chemical reactions easily and also medical instruments, used as alloys for metals, etc.
Platinum Facts for Kids
Platinum was an element that was discovered by the South American people in their artwork and jewellery long before the Spanish came into their lands. Along with Platinum, they made use of Gold alloys as well.
It was an English scientist named William Hyde Wollaston who discovered the purest form of Platinum in 1803.
Platinum is considered one of the most expensive metals since they are very rare to find. In today’s world, the majority of platinum comes from countries like South Africa and Russia.
Apart from platinum, gold is the only metal that is considered more malleable in front of it.
The other elements of the group to which platinum belongs are also known as the Platinum Group Elements.
Since platinum is considered to be a very valuable metal, some awards, honours and superior designations are placed on its name and are called platinum awards.
Summary
Platinum, which is considered to be the third most abundant element in the universe, is also a very precious metal and has properties of ductility and malleability which make it a great investment for currencies. Platinum shows different characteristics such as its rarity and oxidisation. Also, it is considered one of the most expensive metals since many awards are named after its name. Platinum is one of the most important elements of the periodic table since it is rare and helps in different chemical reactions as well. It has some significant properties and usages in our everyday life which helps us to move forward with chemistry easily.
FAQs on Facts About Platinum
1. Why is platinum known as white gold?
Platinum has various chemical and physical properties to it some of which are ductility and malleability. Apart from this, it has a shiny silver appearance with resistance to factors like tarnishing and corrosion and hence makes it a very important metal. Due to such features, it is also known as one of the noble metals on the periodic table.
It is known as white gold majorly due to its shiny silver appearance but is considered gold due to its properties being similar to the properties of gold such as the ability to take different shapes and being able to be drawn in thin wires.
2. Why is gold a more preferred element than platinum?
Platinum is considered to be the only metal that has more significant properties towards ductility and malleability as compared to gold. Even though platinum is a better material than gold, people still tend to prefer gold over platinum since gold was a better-known element throughout the world and came much before platinum did.
Although, since the 19th century, platinum has been considered to be used more and more every day and hence has been making more progress than gold. Hence, it is not completely true that gold is preferred more than platinum.
3. How do you test the purity of platinum?
The purity of platinum is tested by using a Technetium-99m (Tc-99m) isotope as it has a short half-life which means you need to have physical contact with it. Tc-99m is also used in different medical procedures. One of the main reasons for its use is its half-life of 90 minutes. It has various uses due to its rarity and properties. As a cathode in atomic batteries and as a catalyst in the petroleum industry, it helps in different reactions easily.
4. How is platinum made?
Platinum is made by refining elements like copper and nickel.









