




About: Sulfate or Iodine
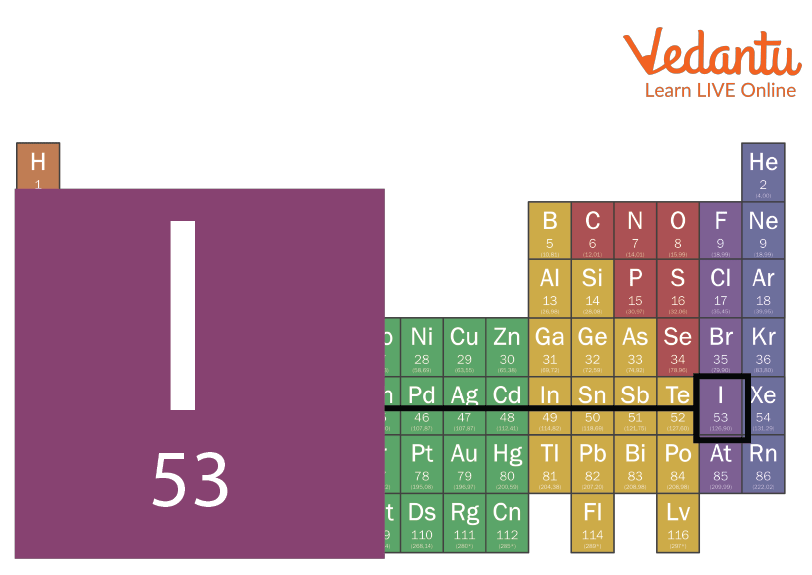
Iodine is a chemical element. The symbol for Iodine is I. It's atomic number is 53. It lies in the 17th group of the periodic table which is known as the halogen group. It is the heaviest of stable halogens. It is a non metal. Iodine was discovered by French chemist Bernard Courtois in 1811. It's name was given by Joseph Louis Gay- Lussac. Read further to know more about iodine, its properties, uses and more. Down below is the image of the iodine periodic table.

Iodine Periodic Table Position
Occurrence of Iodine Element
Iodine is not found in nature. It is found in seawater as iodide ion. It is also found in seaweeds, oysters and cod livers. Iodine is also found in the human body in thyroxine which is produced by the thyroid gland. Natural occurring isotope of iodine is iodine 127, a radioactive isotope is iodine 131. Iodine 129 changes from radioactive form to nonradioactive in millions of years. Iodine occurs in many oxidation states which are iodide, iodate and various periodate anions. Iodate minerals, brackish water from oil and salt wells are the main sources of iodine. The iodine element is a chemical element , a member of the halogen element.

Iodine Crystals
Position of Iodine
Iodine is a non-metal that lies in group 17 that are halogens and period 5. It lies below Bromine (Br) and above Astatine (At), to the right of Tellurium (Te), and to the left of Xenon[Xe]. According to its position, it is non metal and a halogen. Most of the chemical and physical properties of iodine are decided by its position on the periodic table such as metallic or non metallic character, electronegativity, enthalpy, etc.
Physical Properties of Iodine
The physical properties of iodine are discussed below.
Iodine is non-metal and solid at room temperature.
It is dark, shiny, grey-black in colour.
Its melting point is \[{113.5^o}\]C.
Its boiling point is \[{184.35^o}\]C.
It is the most electropositive halogen.
Chemical Properties of Iodine
The chemical properties of iodine are listed below.
Its chemical properties are much similar to fluorine, chlorine and bromine.
It sublimes easily to a deep violet vapour.
Iodine is highly corrosive.
It dissolves slightly in water.
Iodine is a poisonous halogen.
Uses of Iodine
Some of the common uses of iodine are as follows:
Iodide salts are used in pharmaceuticals and disinfectants.
Iodine reduces thyroid hormone and can kill fungus and bacteria.
Iodine is used to make polarising filters for LCDs.
Radioiodine can be a possible treatment option for thyroid cancer.
It also helps the cells to make protein.
The commercial use of iodine is in photography.
Fun Facts About Iodine
The majority of our iodine comes from milk.
Iodine controls our metabolic rate.
Our brains require iodine.
It's a fantastic antiseptic.
Cancer can be cured with radioactive iodine.
It was employed in the creation of the earliest photos.
In order to check for starch, iodine is needed.
It can be used to keep paint from fading.
Solved Questions
1. What is the electronic configuration of iodine?
Ans: The electronic configuration of iodine is [Kr] \[4{{\rm{d}}^{10}}5{{\rm{s}}^2}5{{\rm{p}}^5}\]
2. What is the natural colour of iodine?
Ans: Iodine is nearly black or grey-black in colour.
3. What is iodine on the periodic table?
Ans: Iodine is an atomic number 53 chemical element with the symbol I. Iodine is a solid at room temperature and is categorised as a halogen.
4. Where is iodine on the periodic table?
Ans: Iodine (I) is a chemical element that belongs to Group 17 of the periodic table, also known as the halogen elements.
Conclusion
Iodine is a non-metallic solid element that lies in group 17, which is known as the halogen group, and period 5 of the periodic table. Along with earth's crust, it is found in seawater. It mainly has 3 isotopes naturally and is radioactive. Out of the halogen family, it is the least abundant. It plays an important role in the drug industry. Seaweed contains high concentrations of iodine. It is an essential mineral for human health.
FAQs on Facts About Iodine ( I )
1. What is iodine and what is its chemical symbol?
Iodine is a chemical element with the atomic number 53 and the symbol I. It belongs to the halogen group on the periodic table. At room temperature, it exists as a lustrous, purple-black solid.
2. What are some important examples of iodine's use in daily life?
Iodine has several important applications that we encounter regularly. Some key examples include:
- Antiseptics: Tincture of iodine is a common antiseptic used to clean wounds and prevent infection.
- Dietary Supplements: It is added to table salt (iodised salt) to ensure people get enough for their health.
- Water Purification: Iodine tablets can be used to disinfect water, making it safe to drink.
- Medical Imaging: Certain radioactive isotopes of iodine are used as contrast agents in X-rays and CT scans.
3. Is iodine a metal or a non-metal?
Iodine is classified as a non-metal. However, it displays some properties that are unusual for non-metals, such as a solid state at room temperature and a metallic lustre, which sometimes leads to it being referred to as a metalloid. It is the heaviest of the stable halogens.
4. Which foods are good natural sources of iodine?
Besides iodised salt, iodine is naturally present in several foods. The best sources are often from the sea, including seaweed (like kelp), fish (such as cod and tuna), and other seafood like shrimp. Other good sources include dairy products like milk and yoghurt, as well as eggs.
5. Who is credited with the discovery of iodine?
The discovery of iodine is credited to the French chemist Bernard Courtois in 1811. He accidentally isolated the element while extracting sodium and potassium compounds from seaweed ash. The new element was named 'iodine' from the Greek word 'iodes', meaning violet, due to the colour of its vapour.
6. Why is iodine so important for the human body?
Iodine is a crucial micronutrient because the thyroid gland needs it to produce essential hormones, primarily thyroxine (T4) and triiodothyronine (T3). These hormones regulate the body's metabolism, support bone and brain development during pregnancy and infancy, and control other important bodily functions.
7. What happens if a person has an iodine deficiency?
An iodine deficiency means the thyroid gland cannot produce enough thyroid hormones. This can lead to several health problems, including an enlargement of the thyroid gland known as a goitre. It can also cause hypothyroidism, a condition with symptoms like fatigue, weight gain, and sensitivity to cold. Severe deficiency during pregnancy can impact the baby's brain development.
8. Why does solid iodine turn directly into a gas when heated?
Solid iodine turns directly into a purple vapour without first melting into a liquid. This unique process is called sublimation. It occurs because the forces holding the iodine molecules together in the solid crystal are relatively weak, allowing them to escape directly into the gaseous state when they gain enough energy from heat.
9. How is iodine used to test for the presence of starch?
The starch-iodine test is a common chemical experiment. When a few drops of an iodine solution (like Lugol's iodine) are added to a substance containing starch, a distinctive deep blue-black colour appears. This reaction is highly specific and is used to identify the presence of starch in materials like potatoes, rice, and bread.
10. If seafood is rich in iodine, why is table salt iodised?
While seafood is a great source of iodine, not everyone has access to it or consumes it regularly. Fortifying a common household staple like table salt with iodine is a simple and highly effective public health strategy to ensure that a large population consistently receives the required amount of this essential nutrient, thereby preventing widespread iodine deficiency disorders.









