




What Are the Main Differences Between Evaporation and Condensation?
Remember the last time you were absolutely mesmerised by that one sniff of rose water? How pleasant was the fragrance? Though many types of rose water available on the market are synthetic, natural ones are made from real roses.
If you’re wondering how the aroma of rose petals is infused into water, there are special processes to do that. Before we dive deeper to understand the steps involved in this, it is important to grasp a few concepts.
Rose Water Prepared using Evaporation, and Condensation Processes
What is a Physical Change?
Change in the state of a substance due to change in its environment can be defined as physical change. If that’s a mouthful for you, it’s basically a physical change every time water (a liquid) changes to ice (a solid) and the reverse process. This is because we observe a change in their physical state (liquid to solid or the other way around).
It is interesting to note that physical changes are reversible as these changes do not bring about a change in the chemical nature of the substance

Illustration of a Physical Change
What are the Various Processes that Bring about a Physical Change?
There are many such processes that bring about physical change.
Evaporation is defined as the process by which liquids when given external energy (mostly in the form of heat) convert into gases.
If you rethink how this is related to producing rose water, the water containing rose petals is boiled first, once the boiling point is reached (actual temperature when boiling takes place) the water mixture evaporates (forming rose flavoured vapours).
Condensation is defined as the process by which gases take external energy (reduced temperature) to convert into liquids.
Based on this principle, the rose-flavoured vapours are condensed by their contact with a cold surface. This gives us pure and aromatic rose-infused rose water.
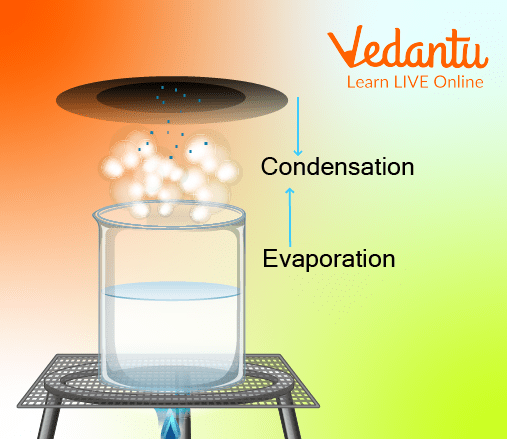
Process of Evaporation followed by Condensation of Water
Evaporation
Evaporation, a word derived from Latin ēvapōrāre meaning “to disperse into vapour” explains what evaporation is all about.
During this process, stable little liquid molecules are given energy in the form of heat. Now, molecules lose their stability when they gain energy because of which these molecules tend to move faster. When this happens, molecules break away from the surface of the liquid. They escape the surface of the liquid to enter the air as gas molecules (Think of evaporating liquid molecules like super-excited children who keep running around as they are too restless to sit.)
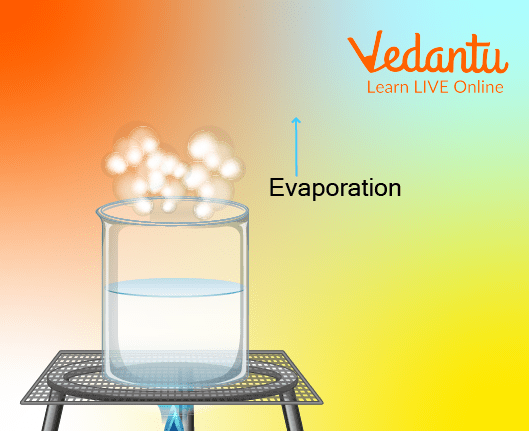
Evaporation of Water (Evaporation Diagram)
Applications of Evaporation in Our Daily Life (Evaporation Examples)
Wet clothes dry under the sun due to evaporation.
Food preservation is possible because of the process of evaporation.
Rain on earth is possible because of the evaporation of water bodies. The escaped water vapours undergo condensation (which we will be discussing in detail later) to produce precipitation.
Salt is produced by the evaporation of saline waters obtained from various natural sources.
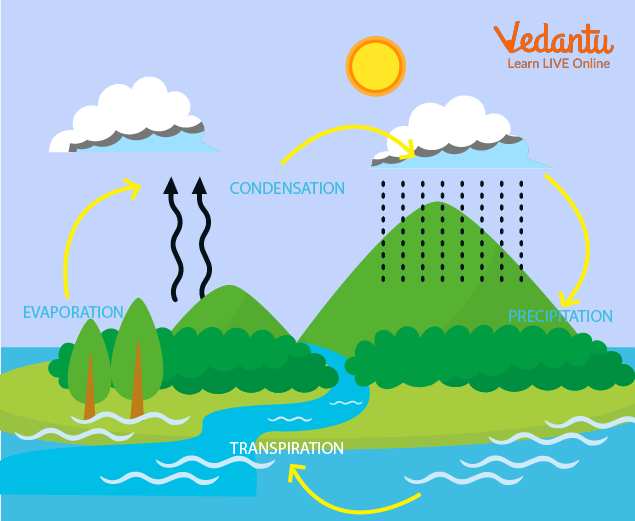
Evaporation's Role in Precipitation
Condensation
Condensation, a word derived from Latin condensate meaning “press close together” is often termed as the opposite of evaporation. Condensation is the process of converting gases back into a liquid with the release of energy.
Here, molecules of escaped gases lose energy when they come in contact with a cold surface. As they lose energy, the molecules begin to rest and settle down. They move closer as they lose energy to convert back into liquids. The energy lost by these rather ‘tired’ molecules appears as heat.
(Condensation in terms of our playful kid's analogy is tired kids all settling down after a long day at play)
Applications of Condensation in Our Daily Life (Condensation Examples)
Evaporated water vapours condense in the clouds to fall as rain or snow. Rain requires both evaporation and condensation.
Pretty clouds themselves are due to condensation of various gases present in the atmosphere.
Condensation of breath on cold windows during winter months.
Water droplets seen on the grass in early mornings are also due to condensation.
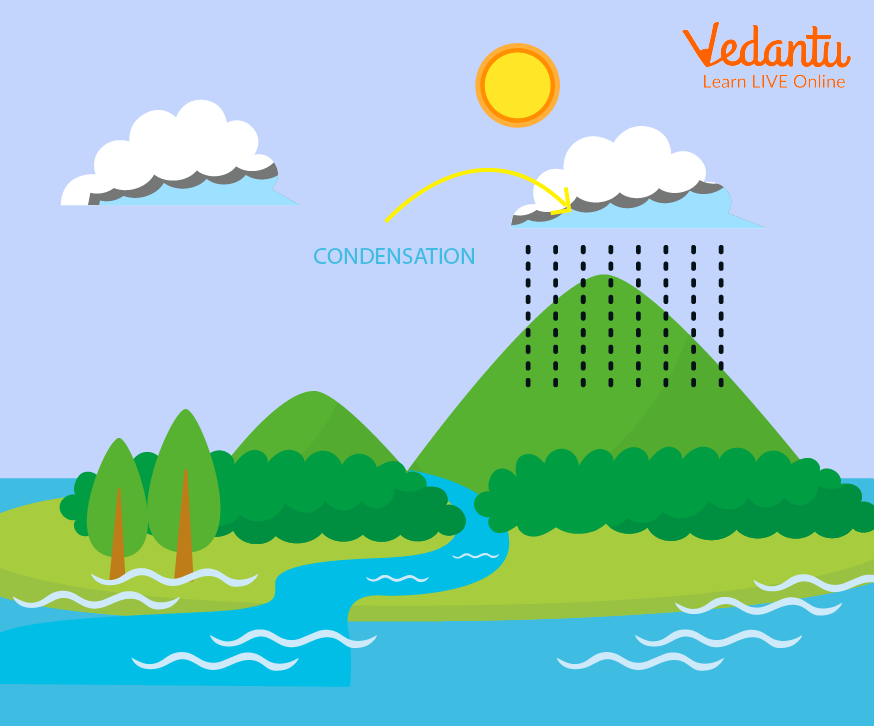
Condensation in Rain Cycle (Condensation Diagram)

Water Droplets on Grass due to Condensation
Difference between Condensation and Evaporation
Summary
Physical change is when a substance changes from one physical state to another without change in its composition. Several processes bring about physical changes like evaporation, condensation, boiling, melting, freezing, etc.,
Evaporation is the process of change in state from liquid to a gaseous state.
Condensation is the process of change in state from a gaseous state to a liquid state.
Evaporation is observed during salt formation from water bodies and Condensation is observed in clouds.
Evaporation and condensation are basically opposite processes of each other.
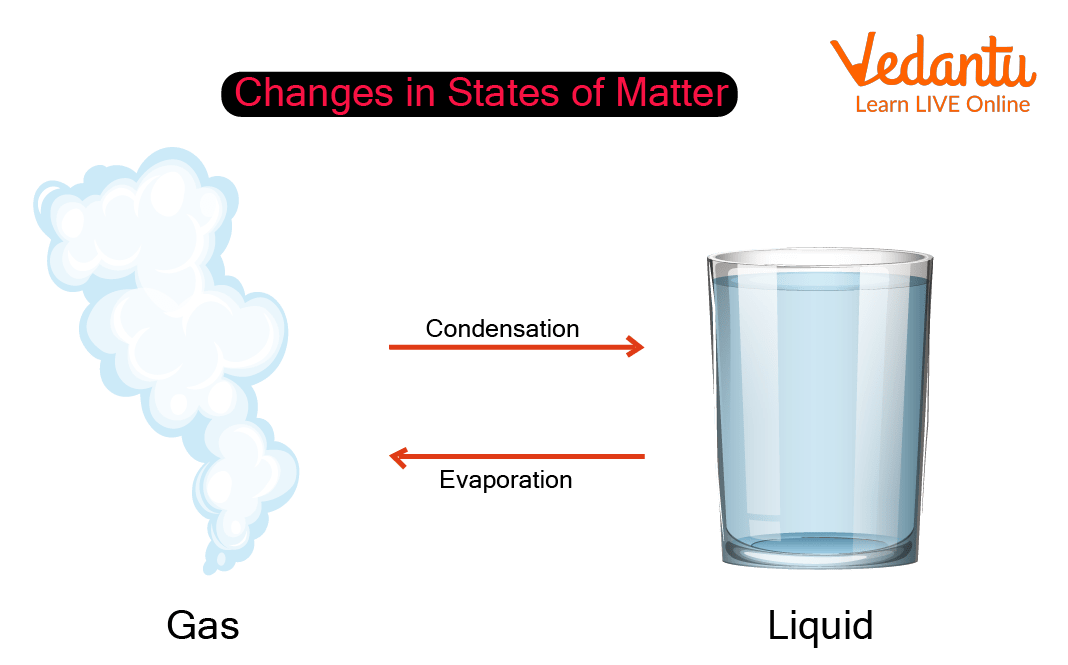
Opposite Reactions of Condensation and Evaporation
FAQs on Evaporation and Condensation Explained for Students
1. What is evaporation in simple terms?
Evaporation is the natural process where a liquid turns into a gas or vapour without having to boil. This happens on the surface of the liquid. A common example is when a puddle of water on the ground slowly disappears on a sunny day.
2. What is condensation and where can we see it?
Condensation is the opposite of evaporation. It's the process where a gas cools down and turns back into a liquid. You can see this when tiny water droplets form on the outside of a cold drink bottle or on a window on a chilly morning.
3. What is the main difference between evaporation and condensation?
The main difference is the direction of the change. Evaporation is when a liquid becomes a gas (like water turning into steam). Condensation is when a gas becomes a liquid (like steam turning back into water droplets). They are opposite parts of the same cycle.
4. How are clouds formed using both evaporation and condensation?
Clouds are a perfect example of these two processes working together. First, the sun's heat causes water from oceans and lakes to evaporate and rise as water vapour. High up in the sky, the air is colder. This cold air causes the water vapour to condense into billions of tiny water droplets, which then form the clouds we see.
5. What factors make evaporation happen faster?
Several things can speed up the rate of evaporation:
- Heat: A liquid will evaporate faster when the temperature is higher.
- Wind: Wind blows away the water vapour above the surface, allowing more liquid to evaporate quickly.
- Surface Area: Water spread out in a wide, flat dish will evaporate faster than the same amount of water in a tall glass.
6. Why do wet clothes dry faster on a windy day?
Wet clothes dry because the water in them evaporates. On a windy day, the wind blows away the moist air surrounding the clothes. This allows more water to evaporate from the fabric more quickly, helping the clothes to dry faster compared to a calm day.
7. How is evaporation different from boiling?
Evaporation is a slow, quiet process that happens only at the surface of a liquid and can occur at any temperature. Boiling is a fast, bubbling process that happens throughout the entire liquid but only at a specific high temperature known as the boiling point.
8. Why is evaporation important for life on Earth?
Evaporation is a crucial part of the Earth's water cycle. It helps move water from oceans and lakes into the atmosphere. This water then falls back as rain, which is essential for drinking water, farming, and for all plants and animals to survive. It also helps cool down the planet's surface.









