




What are Crystals?
rystal is a solid form of particles that exist everywhere around us and comes to use in our daily lives. Here, we will discuss how crystals grow, where do crystals come from, and much more about crystals. Crystals are mainly made up of atoms. Crystal formation takes place when different molecules come and fit together. Crystals are used by us in different areas of our life.
What are Crystals, and How are they formed?
When different atoms, ions, or molecules form a set of designs and are arranged accordingly, it leads to the formation of crystals. These atoms, ions, or molecules repeat themselves to form crystals.
The unit cell is considered to be the base structure or the building block of the crystal form. This unit cell is built of atoms, ions, or molecules that repeat themselves to form a solid structure. This whole process of how crystals are made is called crystallisation.
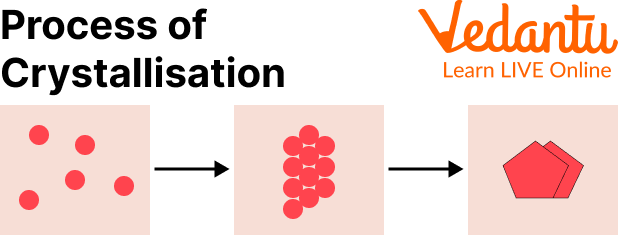
Crystallisation
The crystals are generally formed when some type of liquid starts cooling down and ultimately creates a solid structure. This is the process of how crystals form in rocks. They mainly develop when liquid rocks like magma start to cool down. Different valuable and expensive crystals like a diamond, rubies, etc are formed in nature through this process of crystallisation.
Another process through which crystals can form is through evaporation. When water starts evaporating from a mixture, leaving solids or crystals behind in the mixture. This process is common to form salt crystals and separate them from water.
Some of the characteristics important for the crystal are mentioned below:
They are firm and rigid in structure.
They hold a definite shape that remains fixed.
One cannot compress the crystal structures.
Unique Properties of Crystals
The crystals can be formed in different shapes like triangle squares, rectangles, etc., depending on the structuring of atoms during the initial formation of crystals. Moreover, crystals can have very flat surfaces, which are known as facets.
The smaller molecules which come together while the formation of bigger crystals generally represent the same shape. The smaller structures and the bigger structures remain the same irrespective of the number of ions or atoms involved.
There are seven basic structures involved with the shape of atoms which are mentioned below-
Cubic
Trigonal
Triclinic
Orthorhombic
Hexagonal
Tetragonal
Monoclinic
Apart from the seven mentioned shapes, there are a few interesting shapes that have unique structures.
Interesting and Unique types of Crystal Formation
Snowflake Formation
Ice formation, which comes down from the sky when water freezes in the clouds form the snowflake formation. This only occurs only when snowfall happens and hence is a unique formation.
Quartz
Quartz is a hard mineral and crystal and is considered a gemstone that has a unique structure for a crystal.
Diamond
These are the most valuable and expensive crystals existing on the earth. Apart from being used in pieces of jewellery, diamonds are the type of crystals that are used as tools as well. Diamond is considered to be an allotrope of carbon.
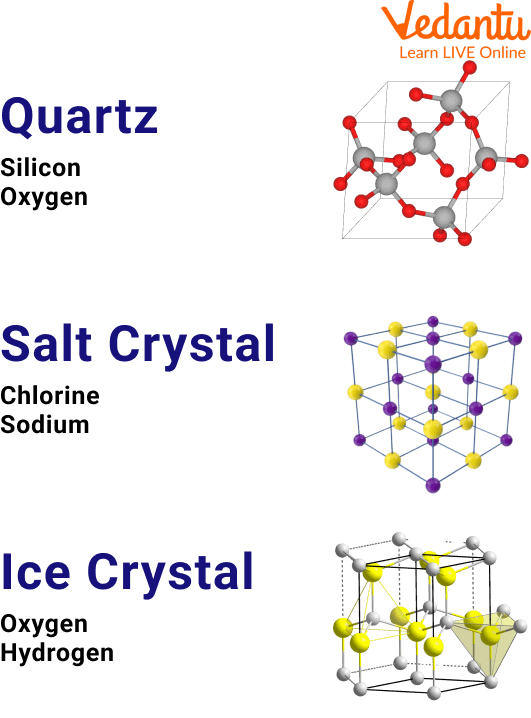
Unique Crystals
Fun Facts of How are Crystals Made
Some of the interesting facts about how do crystals form are as follows:
There is a specific study based on crystal formation and its attributes known as crystallography.
The crystals contain several minute structures invisible to the naked eye, which come together to form a bigger structure that is visible to us in everyday life.
Liquid crystals are also an essential aspect of our everyday life. The computer screens and the television which we look at daily contain liquid crystals in their monitors and screens.
Different types of crystals are very popular in their use in jewellery since they have lustrous properties and a shiny appearance.
Uniquely enough, some living organisms are also capable of producing crystals.
Summary
Crystals are present all around us and are made up of small infinite structures through the process of crystallisation. No one can analyse how crystals are made and occur in nature in different forms. After reading this article you might have gotten the answer to how crystals grow, how crystals form in rocks, and where do crystals come from. I hope this article will help you to understand more about crystals. If you have any doubt do ask in the comments.
FAQs on How do Crystals form?
1. What exactly is a crystal?
A crystal is a solid material where the atoms, molecules, or ions are arranged in a highly ordered, repeating pattern. This internal structure, known as a crystal lattice, extends in all three dimensions and is responsible for the characteristic flat faces and sharp edges seen on many crystals like salt and quartz.
2. How are crystals formed step-by-step?
Crystal formation, or crystallization, generally occurs in two main steps. The first step is nucleation, where a small number of atoms or molecules gather to form a stable, tiny seed crystal. The second step is crystal growth, where more atoms or molecules from the surrounding solution or melt attach to the surface of the seed crystal, causing it to grow larger layer by layer.
3. What are the main ways crystals form in nature?
Crystals form in nature through several key processes. The most common methods include:
- Cooling of magma or lava: As molten rock cools slowly deep within the Earth, atoms have time to arrange into large crystals, forming rocks like granite.
- Evaporation of solutions: When water containing dissolved minerals (like salt water) evaporates, the minerals are left behind and organise into crystals.
- Precipitation from solutions: When a solution becomes supersaturated (overfilled with a substance), the excess substance can solidify into crystals. This happens in places like hot springs and deep-sea vents.
4. What are some common examples of crystals we see in daily life?
You can find many examples of crystals in and around your home. Some of the most common ones are:
- Table Salt (Sodium Chloride): These are tiny cubic-shaped crystals.
- Sugar (Sucrose): The granules you use for sweetening are small crystals.
- Snowflakes: Each one is a unique hexagonal ice crystal.
- Quartz: A common mineral found in sand and used in watches.
- Gemstones: Precious stones like diamonds, rubies, and emeralds are all examples of crystals.
5. Why do crystals have such flat faces and sharp edges?
The flat faces and sharp edges of crystals are a direct external reflection of their perfectly ordered internal structure. The atoms inside are arranged in a repeating, grid-like pattern. As the crystal grows, new atoms can only attach themselves in specific spots that continue this pattern. This orderly, layer-by-layer growth naturally results in smooth, flat surfaces (faces) that intersect at specific, fixed angles, creating the sharp edges.
6. What is the difference between a crystal and a piece of glass?
The key difference is the internal arrangement of their atoms. A crystal has a highly ordered, repeating atomic structure. In contrast, glass is an amorphous solid, which means its atoms are jumbled together in a disordered, random way, similar to the structure of a liquid. This is why glass breaks into curved, sharp fragments, while a crystal tends to break along flat planes that follow its internal structure.
7. Can crystals continue to grow bigger over time? If so, how?
Yes, crystals can grow larger as long as the necessary conditions are maintained. For a crystal to grow, it needs a continuous supply of its building blocks (the same atoms or molecules it's made of) and the right environmental conditions, like temperature and pressure. For instance, a crystal at the bottom of a mineral-rich solution can continue to grow as more molecules from the solution deposit onto its surface, adding to its crystal lattice layer by layer.
8. Besides being beautiful, what are some important industrial uses of crystals?
Crystals are vital to modern technology due to their predictable, ordered structures. Key uses include:
- Silicon Crystals: These are the foundation of all modern electronics, forming the basis for semiconductor chips in computers, smartphones, and solar panels.
- Quartz Crystals: They are piezoelectric, meaning they produce a small electric voltage when squeezed. This property is used to keep precise time in quartz watches and stabilise frequencies in electronic devices.
- Diamond Crystals: Due to their extreme hardness, industrial diamonds are used in cutting, drilling, and grinding tools.









