




Key Properties and Everyday Uses of Arsenic
Symbol of Arsenic: As
The Atomic Number of Arsenic: 33
Atomic Weight: 74.92
Division: Metalloid
Phase at Room Temperature: Solid
Density: 5.727 grams per cm cubed
Melting Point: 817°C, 1503°F
Boiling Point (Sublimation Point): 614°C, 1137°F
Found by: Albertus Magnus in 1250
Arsenic Valence Electrons: 5
Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and non-metal categories. Boron, germanium, silicon, antimony, arsenic, tellurium, and polonium are the seven most widely recognized metalloids.
Metalloids are located between metals and nonmetals. The orange colour on the Periodic table represents metalloids. They form a separating boundary between metals and nonmetals.
Arsenic is the third element in the fifteenth segment of the periodic table. It is classified as a metalloid because it has a few properties like metal and others of non-metal. Arsenic atoms have 33 electrons and 33 protons. Arsenic has 4 valence electrons in the outer shell.

Arsenic
Properties of Arsenic
Arsenic exists in various allotropes. Allotropes are various structures of a similar component. Although they are composed of similar elements, their various structures can have different properties. For example, carbon has the allotropes graphite and diamond.
Arsenic's two most normal allotropes are yellow and metallic grey. Grey arsenic is weak and sparkling strong. Yellow arsenic is delicate and waxy. Yellow arsenic is reactive and extremely harmful. It converts to grey arsenic when presented to light at room temperature. Another allotrope is black arsenic.
Arsenic is normally present at high levels in the groundwater of various nations.
Arsenic is exceptionally harmful in its inorganic form.
Contaminated water utilised for drinking, food planning, and the water system of food crops represents the greatest danger to general well-being from arsenic.
Long-term exposure to arsenic from drinking water and food can cause malignant growth and skin injuries. It has additionally been related to cardiovascular diseases and diabetes. In utero and youth exposure adversely affects the cognitive development of the child.
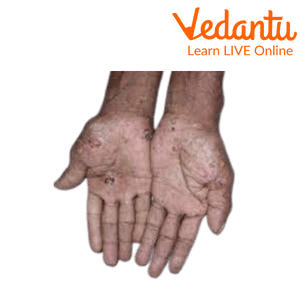
Arsenic Poisoning
Uses of Arsenic
Arsenic has been utilised in the past as a pesticide as well as a wood preservative. Due to natural issues, it is not generally utilised as an insecticide and is being deliberately gotten rid of as a wood additive in the United States.
As a wood preservative, the compound copper arsenate assisted with preventing the wood from decaying and held termites and different insects back from destroying the wood. Arsenic is joined with gallium to create gallium arsenide for use in high-velocity instruments and optoelectronics. Different applications for arsenic incorporate metal compounds and glass making.
How was Arsenic Found?
Arsenic meaning: Arsenic has been referred to about since old times as a component of a compound with sulphur. It is imagined that it was first detached during the Middle Ages by German thinker Albertus Magnus in 1250. Arsenic might have gotten its name from the Greek word "arsenikon" which signifies "yellow shade" or "arsenikos" and which signifies "powerful."
Why is Arsenic Considered Toxic to Humans?
The small molecule of arsenic can easily get into cells, destroys the cells, and interferes with the basic mechanism of the cells. It disturbs cellular respiration and arsenic may interact directly with red cell membranes. This explains the toxicity of arsenic to humans.
How does Arsenic Affect the Environment?
Since arsenic cannot be destroyed, it accumulates in the environment in large amounts and interferes with the environment. Arsenic is emitted by copper, zinc, lead industries, and agriculture as well.
It is also present in plants so it toxifies the plant substances as well. Arsenic is also present in water so it can cause genetic alteration in fishes and the birds which feed on fishes and water organisms can also die due to arsenic poisoning as the fish is decomposed in their bodies.
To prevent this arsenic pollution we need to substitute high arsenic sources such as groundwater with low arsenic sources such as rainwater and treated groundwater. Oxidation, absorption, ion exchange, and membrane techniques are some technologies for arsenic removal.
Arsenic Periodic Table
Arsenic-75 is the only isotope that occurs in nature.
Summary
The symbol of Arsenic is Ar. The atomic number of Arsenic is 33 and Arsenic valence electrons are 5. At the point when arsenic is heated in the air, it combines with oxygen to create arsenic trioxide. Regardless of how harmful arsenic is, a tiny amount is thought of as significant for the strength of animals. Arsenic doesn't melt under standard pressure, however, sublimes directly into gas. It just melts under high pressure. We suggest that you NEVER use, handle, or examine arsenic or its mixtures. It is exceptionally risky. Arsenic is non-combustible but arsenic dust powder when exposed to heat can explode.
FAQs on Is Arsenic a Metalloid?
1. What does it mean for an element to be a metalloid?
A metalloid is a chemical element that has properties in between, or a mixture of, those of metals and non-metals. They are often called semi-metals. For instance, they might look like a metal but be brittle like a non-metal, or act as a semiconductor of electricity.
2. So, is arsenic a metalloid?
Yes, arsenic (As) is a classic example of a metalloid. It sits on the borderline between metals and non-metals in the periodic table and exhibits a mix of their properties. For example, it has a shiny, metallic appearance but is a poor conductor of heat and electricity compared to true metals.
3. What are some other common metalloids besides arsenic?
Several other elements are classified as metalloids. The most commonly recognised ones include:
- Boron (B)
- Silicon (Si)
- Germanium (Ge)
- Antimony (Sb)
- Tellurium (Te)
4. How do the properties of arsenic differ from a true metal or non-metal?
Arsenic perfectly illustrates the in-between nature of metalloids.
- Compared to a metal (like Iron): Arsenic is brittle and will shatter if struck, whereas iron is malleable and strong. Arsenic is also a much poorer electrical conductor.
- Compared to a non-metal (like Sulfur): Arsenic has a metallic lustre (shine), while sulfur is dull. Arsenic can conduct electricity slightly (it's a semiconductor), whereas sulfur is an insulator.
5. Why is arsenic's position on the periodic table important for its classification?
Arsenic is located in Group 15 of the periodic table, right on the diagonal line that separates metals from non-metals. Elements on this line tend to share properties of both sides. As you move down this group from a non-metal (Nitrogen) to a metal (Bismuth), arsenic sits in the middle, representing the transition point, which explains its metalloid character.
6. What is arsenic mainly used for?
Despite its toxicity, arsenic has several important uses. Its primary application is in creating alloys, especially with lead and copper, to make them harder and more durable (for example, in car batteries). It is also used in the manufacturing of semiconductors, pesticides, and wood preservatives.
7. If arsenic is a metalloid, why is it often discussed as a toxic heavy metal?
This is a common point of confusion. While chemically arsenic is a metalloid, the term "heavy metal" is often used more broadly in a toxicological or environmental context to refer to any metallic element that is dense and toxic at low concentrations. Because arsenic shares this high toxicity, it is frequently grouped with elements like mercury, lead, and cadmium in health and safety discussions.
8. How does arsenic typically get into drinking water?
Arsenic can contaminate drinking water through several pathways. The most common is the natural erosion of rocks and soil that contain arsenic-bearing minerals. This arsenic can then dissolve into groundwater. Other sources include industrial pollution from mining, smelting, or the use of agricultural pesticides containing arsenic.









