




An Overview of Class 11 Chemistry Purification Of Chemical Substances By Crystallisation Experiment
Crystallisation is the process through which the atoms or molecules of a substance arrange themselves in a well-defined three-dimensional (3-D) lattice and, consequently, minimise the overall energy of the system. And, when a substance is subjected to crystallisation, its atoms or molecules bind together through well-defined angles. Salt crystallisation is the most practical use of crystallisation, and also the most cost-effective technique to create salt today. It is also used to separate alum crystals from impure samples.
Table of Content
Aim
Apparatus Required
Theory
Procedure
Observations
Result
Precautions
Lab Manual Questions
Viva Questions
Practical Questions
Aim
To determine the crystallisation process of the chemical substances and also create viva questions and answers for this.
Materials Required
Beaker (250 ml)
Sample
Distilled Water
Glass Rod
Wire Gauze
Circular Filter Paper
Funnel
Funnel Stand
China Dish
Tripod Stand
Burner
Crystallising Dish
Watch Glass
Theory
1. Crystallisation Process
Crystallisation is the natural process that takes place when the materials solidify from a liquid, or as they precipitate out from a liquid or gas. This process can be carried out by causing a physical change in temperature, or a chemical change like acidity. This process is carried out based on the shapes and sizes of the molecules involved, along with their chemical properties.
2. Types of Crystallisation
There are mainly three types of crystallisation, and they are based on the methods of formation of crystals. They are Evaporative Crystallisation, Cooling crystallisation and reactive crystallisation or Precipitation.
Procedure
The process of crystallisation involves the following steps:
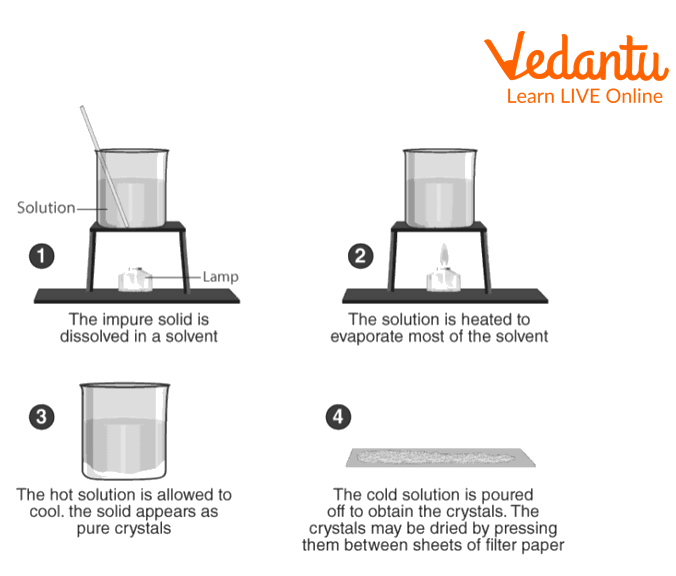
1. Preparation of Solution of the Impure Sample
Take a clean beaker (250 ml) and add the powdered impure sample under consideration in it (~ 6.0 gm).
Add distilled water (25-30 ml) and stir contents gently with the help of a glass rod giving a circular motion.
The solution in the beaker is heated (60°-70 °C) on a wire gauze.
Stir the solution continuously and add more of the impure substance till no more of it dissolves.
2. Filtration of Hot Solution
Take a circular filter paper. First, fold it one-half, then fold it one-fourth. Open the filter paper, three folds on one side and one fold on the other side to get a cone.
Take a funnel and fit the filter paper cone into the funnel so that the upper half of the cone fits well into the funnel, but the lower part remains slightly away from the funnel.
Wet the filter paper cone with a spray of water from a wash bottle, pressing the upper part of the filter paper cone gently against the wall of the funnel with the thumb.
Place the funnel on a funnel stand and place a clean China dish below the funnel for the collection of the filtrate. To avoid splashing the filtrate, adjust the funnel so that its stem touches the wall of the dish.
Hold a glass rod in a slanting position in your hand or with a precaution that the lower end of the rod should reach into the filter paper cone but it does not touch it. Pour the solution along the glass rod. The filtrate passes through the filter paper and is collected into the China dish placed below. The insoluble impurities are left behind on the filter paper.
3. Concentration of Filtrate
Place the dish containing the clear filtrate over wire gauze, kept over a tripod stand and heat it gently (Do not boil). Stir the solution with a glass rod. This is done to ensure uniform evaporation and to prevent the formation of a solid crust.
When the volume of the solution is reduced to one-half, take out a drop of the concentrated solution on one end of the glass rod and cool it by blowing air. The formation of a thin crust indicates that the crystallisation point has been reached.
Stop heating by removing the burner.
4. Cooling the Concentrated Solution
Pour the concentrated solution into a crystallising dish. (It is a thin-walled, shallow glass dish with a flat bottom and vertical sides. It has a spout to pour off the mother liquor).
Cover the dish with a watch glass and keep it undisturbed.
As the solution cools, crystals separate. The concentrated solution is cooled slowly for a better yield of the crystals.
Sometimes the China dish containing the concentrated solution is cooled by placing it on a beaker filled to the brim with cold water. Cooling may also be done by keeping the China dish in the open air, depending upon the weather conditions.
5. Separation and Drying of Crystals
Decant off the mother liquor and wash the crystals with cold water or alcohol.
Dry the crystals by pressing them gently between the sheets of filter paper. The crystals can be dried by spreading them on a porous plate for some time or by placing the crystals in a vacuum desiccator.
Crystals have definite geometry and a definite shape. Copper sulphate crystals are formed in triclinic shape, potash alum comes out in octahedral geometry. Potassium nitrate crystals are needle-like, and ferrous sulphate has a monoclinic shape.
Crystallisation Process
Observations
There are certain steps involved in the process of crystallisation. And they are listed below:-
Preparation of solution of the impure substance or sample.
Filtration of hot solution.
The concentration of filtrate.
Cooling the concentrated solution.
Separation and drying of crystals.
Result
The crystallisation process is used to purify the impure samples involving various steps and finally, with the formation of a thin-crust crystallisation point reached and the sample gets purified.
Precautions
Ensure that the crystals are washed well.
Avoid overheating the solution.
The solution should be cooled slowly and do not use any rapid cooling procedures.
The filtrate should be evaporated slowly by gentle heating of the solution.
The burner should be handled with care.
Lab Manual Questions
1. Give an example of a saturated solution.
Ans: Soda is an example of a saturated solution of carbon dioxide in water.
2. Crystallisation is used to purify liquids, true or false.
Ans: Crystallisation is used to purify solids, not liquids. So, the above statement is false.
3. Which technique is used for removing insoluble impurities from the solution during crystallisation?
Ans: Filtration is the process through which insoluble impurities are removed from the solution during crystallisation.
4. Crystallisation is based on the_.
Ans: Crystallisation is based on the difference in the solubility of the compound and the impurities in a suitable solvent.
Viva Questions
1. What is solubility?
Ans: Solubility is defined as the amount of the solute that when dissolved in 100 grams of the solvent provides a saturated solution.
2. What is filtration?
Ans: Filtration is defined as the process of separating insoluble substances by passing the solution through a filter paper.
3. Define the term ‘crystallisation’.
Ans: The substances when present in well-defined geometrical shapes are called crystals. These are formed when a hot saturated solution of salt is allowed to cool slowly and undisturbed. This process is termed crystallisation.
4. Explain a saturated solution.
Ans: A solution in which no more of the solute can be dissolved at a particular temperature is known as a saturated solution.
5. What is the characteristic of crystals?
Ans: Crystals have well-defined geometry and shape.
6. What is green vitriol?
Ans: It is hydrated ferrous sulphate FeSO4.7H2O.
7. What is meant by the term ‘water of crystallisation’?
Ans: The water of crystallisation is the definite number of water molecules that is present in a loose combination with one formula unit of the compound.
8. Why is the solution not heated to dryness to get crystals?
Ans: Heating the solution to dryness will not remove soluble impurities, and crystals of very poor quality are obtained.
9. Why is the hot saturated solution not cooled suddenly?
Ans: By allowing the saturated solution to cool slowly, crystals grow in size. It helps in their better separation as units, rather than giving a messy substance with no proper geometry.
Practical Questions
One of the most common solvents used for crystallisation is
Water
Normal saline
Alcohol
Sulphuric acid
Ans: Water is the most common solvent used in crystallisation.
Crystals are collected by Gooch crucible with the help of:
Water bath
Force
Centrifuge
Vacuum pump
Ans: Crystals are collected by the Gooch crucible with the help of a vacuum pump.
Crystals are dried with the help of:
Dryer
Autoclave
Filter paper
Fan
Ans: Crystals are dried with the help of filter paper.
The insoluble impurities are removed by:
Filtration
Drying
Heating
Cooling
Ans: The insoluble impurities are removed by the process of filtration.
The solvent should dissolve a large amount of solute at:
Cold temperature
Hot temperature
Melting point
Boiling point
Ans: The solvent should dissolve a large amount of solute at boiling point.
Which of the following is crystallisation?
Solid-solid separation
Solid-liquid separation
Solid-gas separation
Liquid-gas separation
Ans: Crystallisation is the solid-liquid separation.
Which of the following is an example of a crystallisation process?
Purification of seawater
Purification of alum
Separation of gases from the air
None
Ans: The purification of alum is an example of the crystallisation process
Which of the following is known as mother liquor?
Solvent
Solute
Filtrate
Solution
Ans: The filtrate is also known as mother liquor.
Conclusion
From the above experiment, we can conclude that crystallisation is the process used for the purification of chemical substances. This process involves five major steps and those steps are preparation of solution of the impure substance or sample, filtration of the hot solution, concentration of the filtrate, cooling of the concentrated solution and Separation and drying of crystals.
FAQs on Class 11 Chemistry Purification Of Chemical Substances By Crystallisation Experiment
1. Why is preparing a saturated solution considered an important first step for purifying a substance by crystallisation?
Preparing a saturated solution is a crucial first step because it ensures the maximum amount of the impure solid is dissolved in the minimum amount of solvent at a high temperature. When this solution is cooled, the solubility of the substance decreases sharply, forcing the pure substance to crystallise out while the soluble impurities remain dissolved in the solvent (mother liquor). This maximises the yield of pure crystals.
2. What is the specific purpose of hot filtration in a crystallisation experiment for Class 11 Chemistry?
Hot filtration is a critical step performed to remove insoluble impurities from the solution while it is still hot. If the solution were allowed to cool before filtering, the desired substance would also start to crystallise prematurely along with the impurities, leading to an impure product and a lower yield. The filtration must be done quickly to prevent this premature crystallisation in the funnel itself.
3. What are the key steps for obtaining pure crystals of copper sulphate from an impure sample, as per the CBSE 2025-26 practical syllabus?
The key steps for purifying an impure sample of copper sulphate (CuSO₄) are:
- Preparation of Solution: Dissolve the impure sample in a minimum amount of water in a beaker and heat gently.
- Hot Filtration: Filter the hot solution to remove insoluble impurities like dust or sand.
- Concentration: Heat the filtrate gently to evaporate excess water until the crystallisation point is reached. This is checked by dipping a glass rod in the solution and blowing on it; a thin crust forms on the rod.
- Cooling: Allow the concentrated solution to cool down slowly and undisturbed. Pure blue crystals of copper sulphate will separate out.
- Separation and Washing: Decant the mother liquor and wash the crystals with a small amount of cold water or ethanol to remove any adhering impurities.
- Drying: Dry the crystals by pressing them gently between sheets of filter paper.
4. Why is slow cooling essential for obtaining well-defined crystals?
Slow cooling is essential because it allows the particles (ions or molecules) sufficient time to arrange themselves into a well-defined, ordered, and stable crystal lattice. Rapid cooling traps impurities within the crystal structure and leads to the formation of small, irregular, and imperfect crystals. For obtaining large and pure crystals, a slow and undisturbed cooling process is a key requirement.
5. From an exam perspective, why is crystallisation considered a better purification technique than simple evaporation?
Crystallisation is superior to evaporation for purification for two main reasons:
- Separation of Impurities: In crystallisation, soluble impurities remain behind in the mother liquor as the pure substance crystallises out. In evaporation, the solvent is completely removed, leaving behind both the pure substance and any soluble impurities, so no purification occurs.
- Product Integrity: Some substances, like copper sulphate, decompose or lose their water of crystallisation upon strong heating to dryness, which is what happens in evaporation. Crystallisation avoids this by using gentle heating and controlled cooling.
6. What are some common errors a student might make during a crystallisation viva experiment, and how can they be avoided?
Some common errors in the crystallisation of a substance like alum or copper sulphate include:
- Using too much solvent: This results in a very dilute solution, leading to a poor yield of crystals upon cooling. Always use the minimum amount of solvent required.
- Overheating the solution: Concentrating the solution too much can lead to a solid mass instead of crystals. Stop heating once the crystallisation point is reached.
- Cooling the solution too quickly: This forms small, impure crystals. The beaker should be allowed to cool slowly at room temperature.
- Improper washing of crystals: Washing with hot water will dissolve the crystals, reducing the yield. Always wash with a very small amount of cold solvent.
7. What is 'seeding' in the context of crystallisation, and why might it be an important technique?
Seeding is the process of adding a tiny, pure crystal of the same substance (a 'seed crystal') to a supersaturated solution to initiate the process of crystallisation. It might be necessary when a solution is supersaturated but crystallisation does not begin on its own due to a lack of nucleation sites. The seed crystal provides a pre-formed lattice surface onto which more molecules can deposit, triggering the formation of larger crystals.
8. What is 'mother liquor', and is it just a waste product in the crystallisation process?
The mother liquor is the liquid solution that remains after the crystals have been separated from the saturated solution. While it contains soluble impurities, it is also saturated with the dissolved pure substance at that lower temperature. Therefore, it is not simply waste; by further evaporating the mother liquor, a second, less pure crop of crystals can often be obtained, which is an important consideration for maximising the overall yield in a chemical process.
























