
Write the formula of the following compounds by criss cross method
A.Ammonium carbonate
B.Calcium bicarbonate
C.Ferric phosphate
D.Potassium sulfate
E.Sodium zincate
Answer
573.3k+ views
Hint: We have to know that crisscross method is one of the methods to write the chemical formula of the ionic compound. In the criss cross method, we have to cross over the numerical value of each of the ions to become the subscript of another ion. We have to drop the signs of the charges.
Complete answer:
We know that the crisscross method is used to determine the chemical formula. We can write the chemical formula of the ionic compound by the following steps using criss cross method,
1.We have to write symbols and charges of the anion and cation. The cation is written first, and then anion is written.
2.We have to transpose the number of positive charges to become the subscript of the anion and the number of negative charges to become the subscript of the cation.
3.We have to reduce to the smallest ratio.
4.The final chemical formula has to be written and the subscript that has 1 is left off.
5.If there is only one of the polyatomic ions, the parentheses are left off.
i.Ammonium carbonate
In ammonium carbonate, the cation is ammonium ion and anion is carbonate ion.
The symbol and charge of ammonium ion is $N{H_4}^ + $.
The symbol and charge of carbonate ion is $C{O_3}^{2 - }$.
We have to interchange the number of positive charges and the number of negative charges. The subscript of ammonium ion becomes ${\left( {N{H_4}} \right)_2}$ and the subscript of carbonate ion becomes $C{O_3}$.
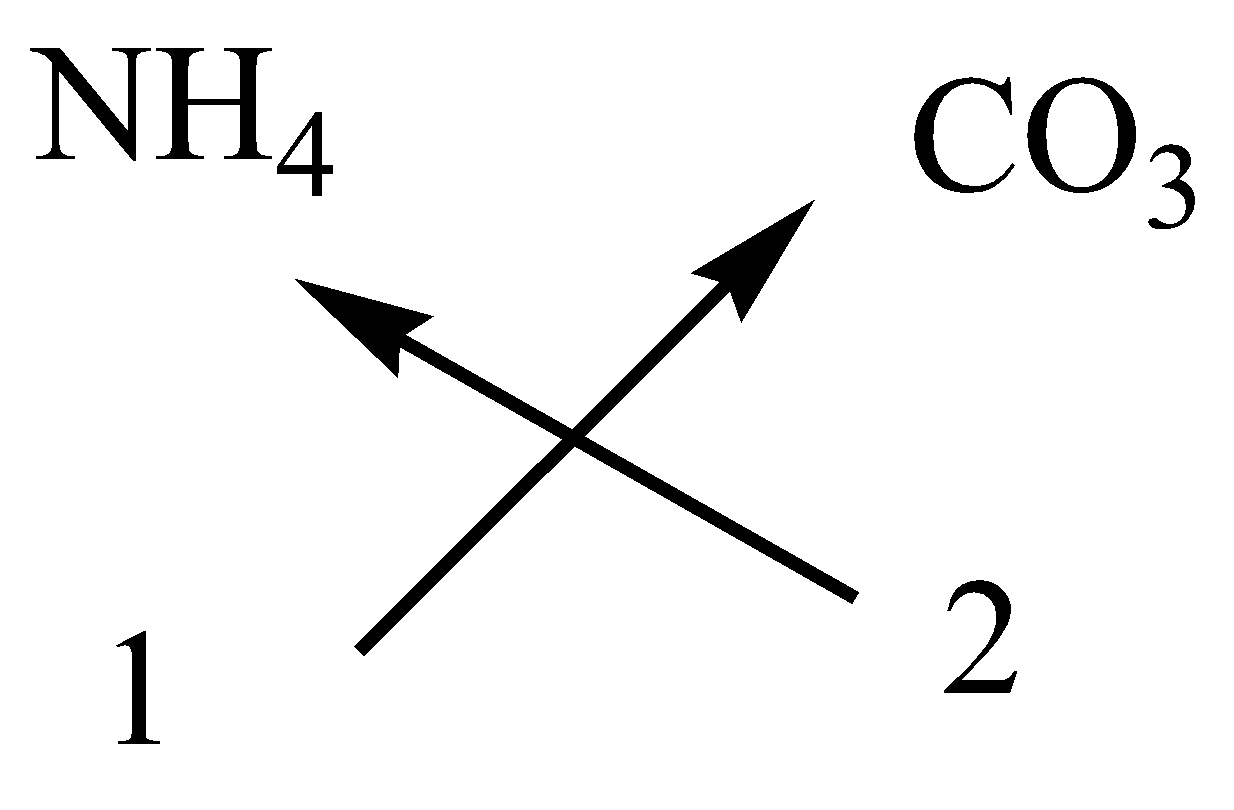
The formula of ammonium carbonate is ${\left( {N{H_4}} \right)_2}C{O_3}$.
i.Calcium bicarbonate
In calcium bicarbonate, the cation is calcium ion and anion is bicarbonate ion.
The symbol and charge of calcium ion is $C{a^{2 + }}$.
The symbol and charge of bicarbonate ions is $HC{O_3}^ - $.
We have to interchange the number of positive charges and the number of negative charges. The subscript of calcium ion becomes $\left( {Ca} \right)$ and the subscript of bicarbonate ion becomes${\left( {HC{O_3}} \right)_2}$.
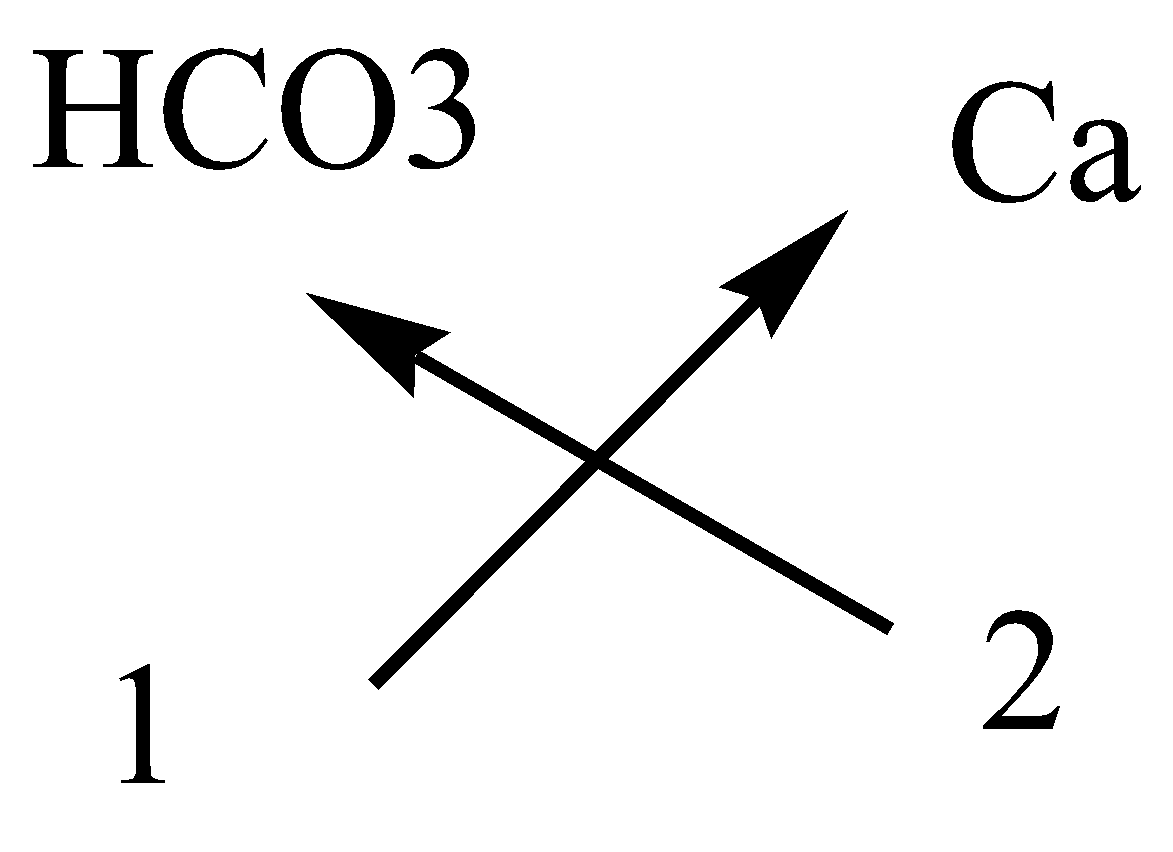
The formula of calcium bicarbonate is $Ca{\left( {HC{O_3}} \right)_2}$.
ii.Ferric phosphate
In ferric phosphate, the cation is ferric ion and anion is phosphate ion.
The symbol and charge of ferric ion is $F{e^{3 + }}$.
The symbol and charge of phosphate ion is $P{O_4}^{3 - }$.
We have to interchange the number of positive charges and the number of negative charges. The subscript of ferric ion becomes ${\left( {Fe} \right)_3}$ and the subscript of phosphate ion becomes ${\left( {P{O_4}} \right)_3}$.
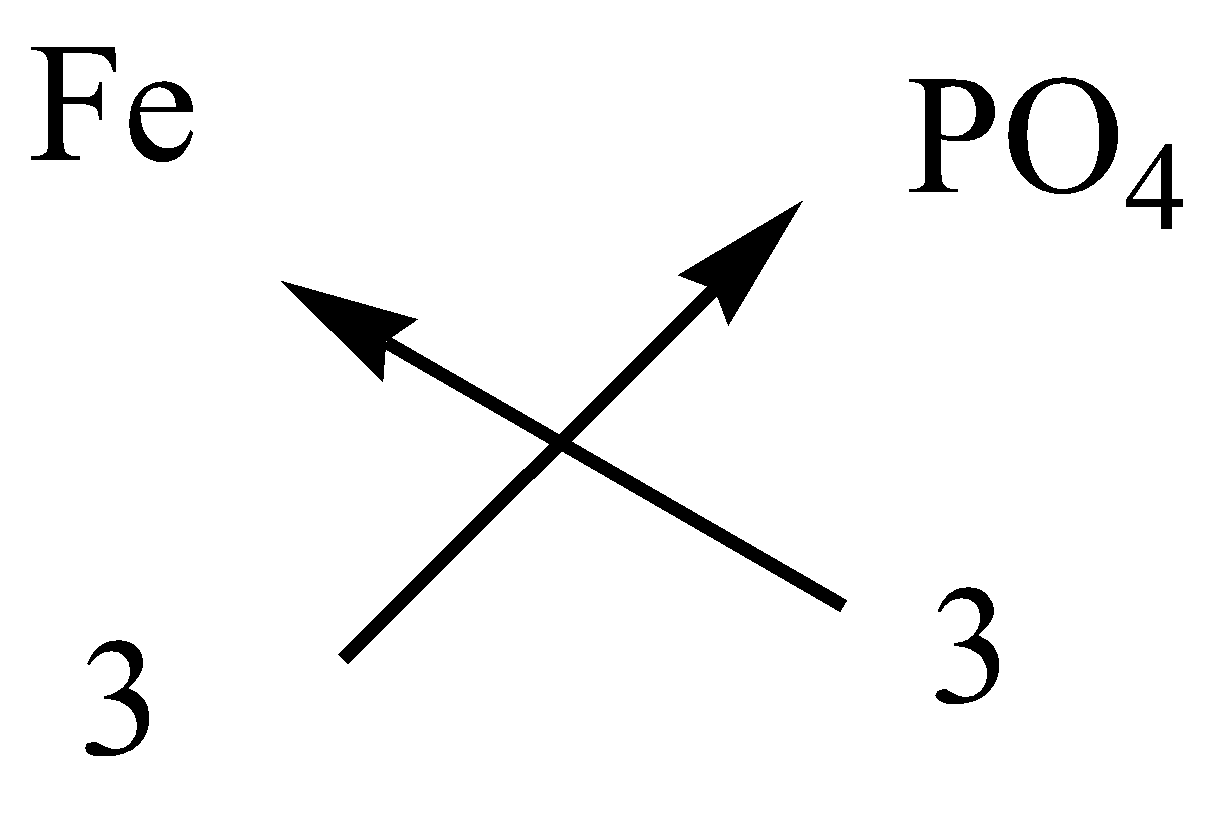
We have to reduce the ratios to get the chemical formula of ferric phosphate.
The formula of ferric phosphate is $FeP{O_4}$.
iii.Potassium sulfate
In potassium sulfate, the cation is potassium ion and anion is sulfate ion.
The symbol and charge of potassium ion is ${K^ + }$.
The symbol and charge of sulfate ion is $S{O_4}^{2 - }$.
We have to interchange the number of positive charges and the number of negative charges. The subscript of potassium ion becomes ${\left( K \right)_2}$ and the subscript of sulfate ion becomes$\left( {S{O_4}} \right)$.
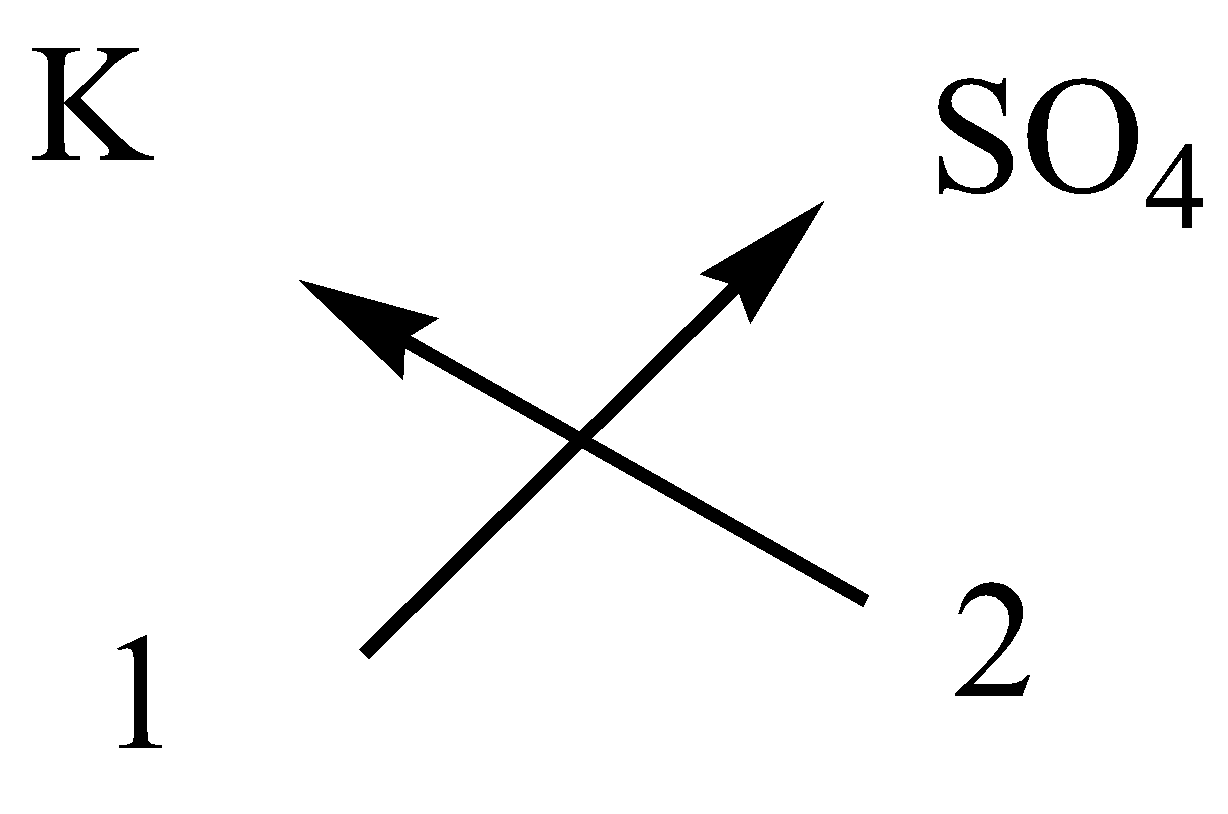
The formula of potassium sulfate is ${K_2}S{O_4}$.
iv.Sodium zincate
In potassium sulfate, the cation is sodium ion and anion is zincate ion.
The symbol and charge of sodium ion is $N{a^ + }$.
The symbol and charge of zincate ion is $Zn{\left( {OH} \right)_4}^{2 - }$.
We have to interchange the number of positive charges and the number of negative charges. The subscript of sodium ion becomes ${\left( {Na} \right)_2}$ and the subscript of zincate ion becomes$\left( {Zn{{\left( {OH} \right)}_4}} \right)$.
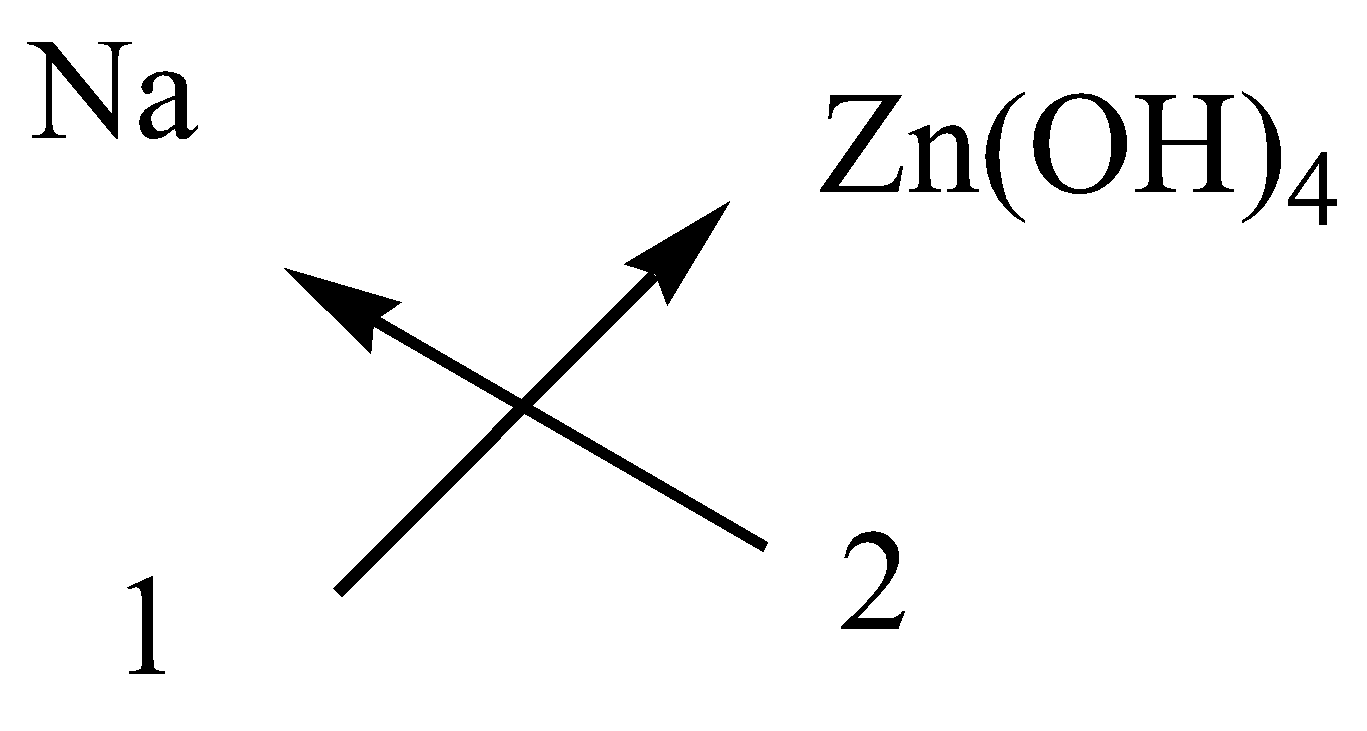
Image created in chemdraw by SME.
The formula of sodium zincate is $N{a_2}Zn{\left( {OH} \right)_4}$.
Note:
We can write the chemical formula of compounds by alternate method. The steps to write the chemical formula of compound by alternate method are discussed below:
We have to write the symbol and charge of cation and anion.
Using a multiplier we have to make the total charges of the anion and cation equal to each other.
The multipliers are used as subscripts for each ion.
The final formula is written and charges are left out and the subscripts and charges that are 1 are left out.
Complete answer:
We know that the crisscross method is used to determine the chemical formula. We can write the chemical formula of the ionic compound by the following steps using criss cross method,
1.We have to write symbols and charges of the anion and cation. The cation is written first, and then anion is written.
2.We have to transpose the number of positive charges to become the subscript of the anion and the number of negative charges to become the subscript of the cation.
3.We have to reduce to the smallest ratio.
4.The final chemical formula has to be written and the subscript that has 1 is left off.
5.If there is only one of the polyatomic ions, the parentheses are left off.
i.Ammonium carbonate
In ammonium carbonate, the cation is ammonium ion and anion is carbonate ion.
The symbol and charge of ammonium ion is $N{H_4}^ + $.
The symbol and charge of carbonate ion is $C{O_3}^{2 - }$.
We have to interchange the number of positive charges and the number of negative charges. The subscript of ammonium ion becomes ${\left( {N{H_4}} \right)_2}$ and the subscript of carbonate ion becomes $C{O_3}$.
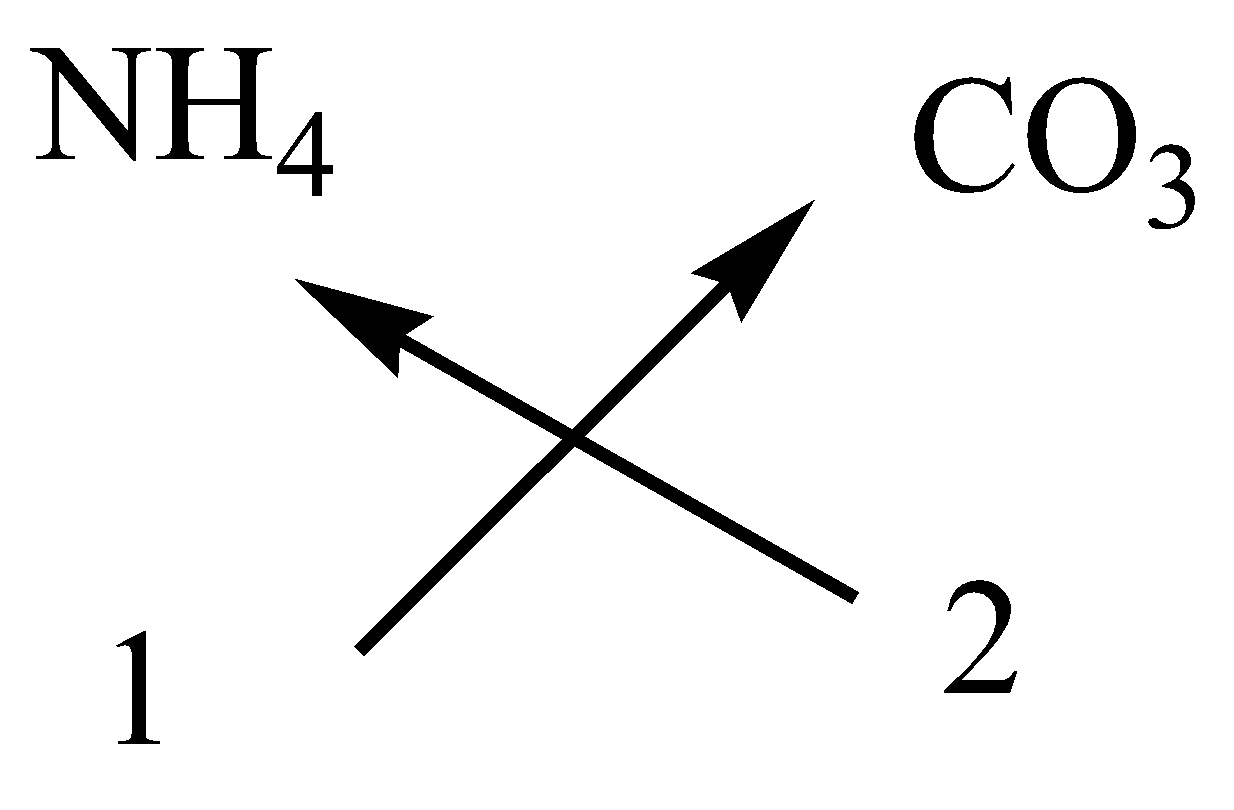
The formula of ammonium carbonate is ${\left( {N{H_4}} \right)_2}C{O_3}$.
i.Calcium bicarbonate
In calcium bicarbonate, the cation is calcium ion and anion is bicarbonate ion.
The symbol and charge of calcium ion is $C{a^{2 + }}$.
The symbol and charge of bicarbonate ions is $HC{O_3}^ - $.
We have to interchange the number of positive charges and the number of negative charges. The subscript of calcium ion becomes $\left( {Ca} \right)$ and the subscript of bicarbonate ion becomes${\left( {HC{O_3}} \right)_2}$.

The formula of calcium bicarbonate is $Ca{\left( {HC{O_3}} \right)_2}$.
ii.Ferric phosphate
In ferric phosphate, the cation is ferric ion and anion is phosphate ion.
The symbol and charge of ferric ion is $F{e^{3 + }}$.
The symbol and charge of phosphate ion is $P{O_4}^{3 - }$.
We have to interchange the number of positive charges and the number of negative charges. The subscript of ferric ion becomes ${\left( {Fe} \right)_3}$ and the subscript of phosphate ion becomes ${\left( {P{O_4}} \right)_3}$.

We have to reduce the ratios to get the chemical formula of ferric phosphate.
The formula of ferric phosphate is $FeP{O_4}$.
iii.Potassium sulfate
In potassium sulfate, the cation is potassium ion and anion is sulfate ion.
The symbol and charge of potassium ion is ${K^ + }$.
The symbol and charge of sulfate ion is $S{O_4}^{2 - }$.
We have to interchange the number of positive charges and the number of negative charges. The subscript of potassium ion becomes ${\left( K \right)_2}$ and the subscript of sulfate ion becomes$\left( {S{O_4}} \right)$.
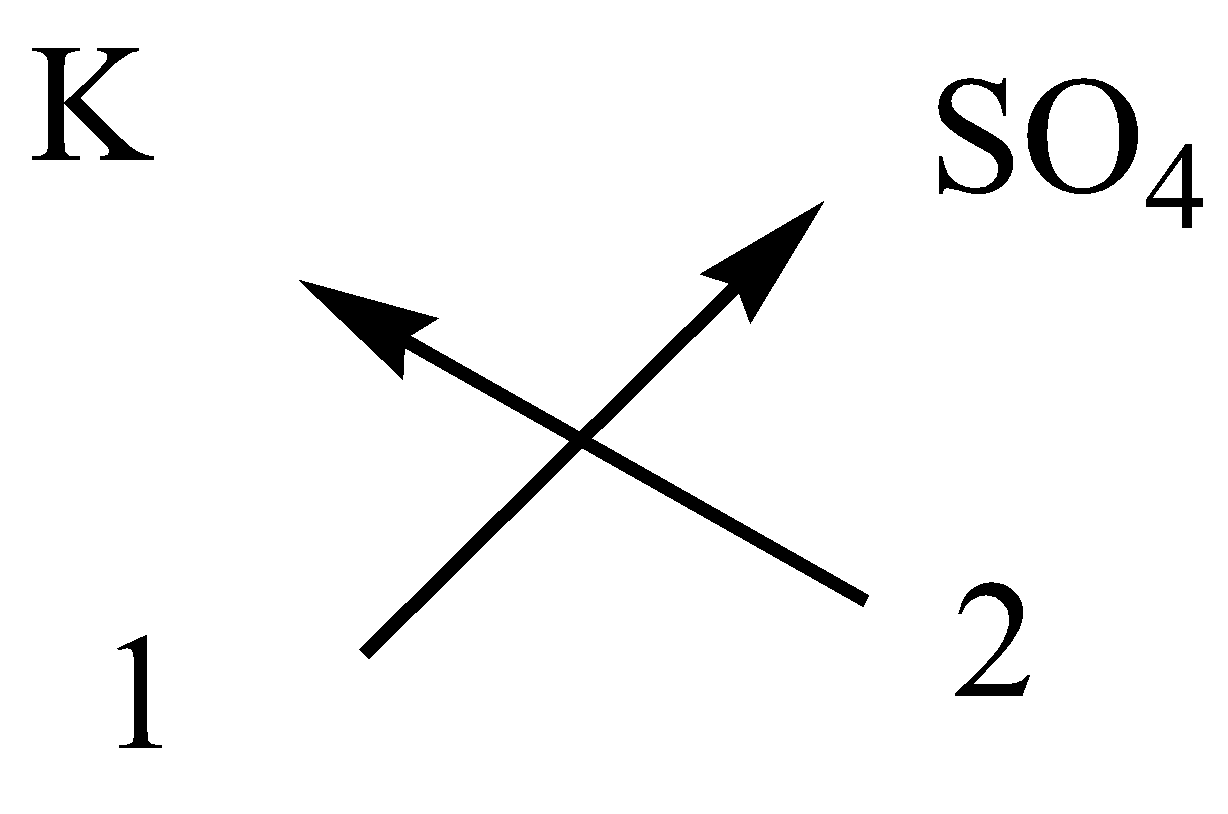
The formula of potassium sulfate is ${K_2}S{O_4}$.
iv.Sodium zincate
In potassium sulfate, the cation is sodium ion and anion is zincate ion.
The symbol and charge of sodium ion is $N{a^ + }$.
The symbol and charge of zincate ion is $Zn{\left( {OH} \right)_4}^{2 - }$.
We have to interchange the number of positive charges and the number of negative charges. The subscript of sodium ion becomes ${\left( {Na} \right)_2}$ and the subscript of zincate ion becomes$\left( {Zn{{\left( {OH} \right)}_4}} \right)$.

Image created in chemdraw by SME.
The formula of sodium zincate is $N{a_2}Zn{\left( {OH} \right)_4}$.
Note:
We can write the chemical formula of compounds by alternate method. The steps to write the chemical formula of compound by alternate method are discussed below:
We have to write the symbol and charge of cation and anion.
Using a multiplier we have to make the total charges of the anion and cation equal to each other.
The multipliers are used as subscripts for each ion.
The final formula is written and charges are left out and the subscripts and charges that are 1 are left out.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




