
What is White Phosphorus stored in?
a.Ether
b.Water
c.Alcohol
d.Kerosene Oil
Answer
596.1k+ views
Hint: White Phosphorus is not a stable allotrope of Phosphorus and is highly reactive to its surroundings.
Step-by-Step Solution:
Phosphorus (Chemical Symbol: P, Z = 15) mainly exists in three major forms: White, Red and Black (all of which have numerous allotropes of their own.)
1.White Phosphorus is a waxy white solid consisting of tetrahedral $P_4$ molecules which is often found to be tinted yellowish when impure.
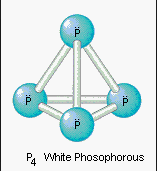
It is an extremely volatile substance which is very reactive and spontaneously combusts in the presence of air, reacting with Oxygen to form Phosphorus Decaoxide $(P_4O_{10})$
$P_4\text{ + }5O_2\text{ }\xrightarrow{{}}\text{ }P_4O_{10}$
and results in chemiluminescence. White Phosphorus is also an extremely toxic substance, harmful in any amount over 0.1 mg.
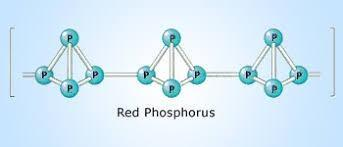
2.Red Phosphorus is an unreactive red powder form of Phosphorus which exists in a polymeric chain of tetrahedral $P_4$ molecules. While Red Phosphorus is unreactive itself, it can be converted into White Phosphorus by subjecting it to either heat, sunlight or friction.
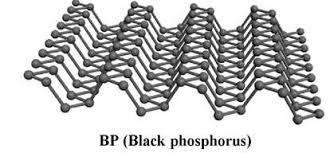
3.Black Phosphorus is the least reactive form of Phosphorus and has little value, it can however be converted into White Phosphorus when subjected to heat under pressure. It exists in the form of an orthorhombic honeycomb-like structure like as follows:
To avoid this explosive reaction with the air which can result in significant injury if carried out without significant precaution, White Phosphorus is stored in Water (a solvent it is insoluble in) to ensure that no chemical reaction and resultant combustion takes place.
Therefore, the answer is b) Water.
Note: R- stands for an alkyl group (ie $C{{H}_{3}},\text{ }C{{H}_{3}}-C{{H}_{2}},$ etc).
Step-by-Step Solution:
Phosphorus (Chemical Symbol: P, Z = 15) mainly exists in three major forms: White, Red and Black (all of which have numerous allotropes of their own.)
1.White Phosphorus is a waxy white solid consisting of tetrahedral $P_4$ molecules which is often found to be tinted yellowish when impure.

It is an extremely volatile substance which is very reactive and spontaneously combusts in the presence of air, reacting with Oxygen to form Phosphorus Decaoxide $(P_4O_{10})$
$P_4\text{ + }5O_2\text{ }\xrightarrow{{}}\text{ }P_4O_{10}$
and results in chemiluminescence. White Phosphorus is also an extremely toxic substance, harmful in any amount over 0.1 mg.
2.Red Phosphorus is an unreactive red powder form of Phosphorus which exists in a polymeric chain of tetrahedral $P_4$ molecules. While Red Phosphorus is unreactive itself, it can be converted into White Phosphorus by subjecting it to either heat, sunlight or friction.

3.Black Phosphorus is the least reactive form of Phosphorus and has little value, it can however be converted into White Phosphorus when subjected to heat under pressure. It exists in the form of an orthorhombic honeycomb-like structure like as follows:

To avoid this explosive reaction with the air which can result in significant injury if carried out without significant precaution, White Phosphorus is stored in Water (a solvent it is insoluble in) to ensure that no chemical reaction and resultant combustion takes place.
Therefore, the answer is b) Water.
Note: R- stands for an alkyl group (ie $C{{H}_{3}},\text{ }C{{H}_{3}}-C{{H}_{2}},$ etc).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




