
Which of the following statements is true about this molecule?
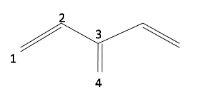
(a) C1-C2 and C3-C4 bonds are of same bond length
(b) C1-C2 bond is shorter than C3-C4 bond
(c) C1-C2 bond is longer than C3-C4 bond
(d) C1-C2 and C3-C4 bonds are of same bond length

Answer
533.7k+ views
Hint: Bonds are responsible for holding atoms in a molecule. The electrons are responsible for formation of various bonds. The length of bonds depends upon various factors. One such factor is bond order, that defines the length of any bond. The bond order is the amount of bonds present.
Complete answer: Bond length of any bond in an atom is the measure of between the nuclei of the bonded atoms. Some factors that affect bond length of an atom are:
-Size of the atom: A greater size atom has more length of bond.
-Multiplicity of a bond: Greater the multiplicity of the bonds less is the bond length. Like a compound with triple bond has less bond length, then the compound with double bonds, while a compound with single bonds has greatest bond length.
-Types of hybridization: S orbital has a small size, so, more is the s character, and less is the bond length.
- Delocalization of electrons through resonance also affects the size of bonds.
Since, the compound contains unsaturation, we will see the resonance factor. The resonating structures of the compound are,
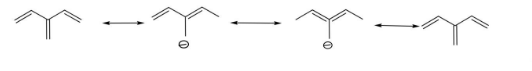
We can see that C1-C2 are having a partial double bond character throughout the delocalization of electrons, while C3-C4 are having a single bond character.
As stated before, that multiplicity of bonds reduces the bond length, while the bond length of a single bond is more, so, due to the resonating structures, C1-C2 has less bond length than C3-C4.
Hence, option (b), C1-C2 bond is shorter than C3-C4 bond is correct.
Note: Bond order which is the number of bonds present also affects the length of bonds. More bond order means less bond length, this factor is also related to resonating structures.
Complete answer: Bond length of any bond in an atom is the measure of between the nuclei of the bonded atoms. Some factors that affect bond length of an atom are:
-Size of the atom: A greater size atom has more length of bond.
-Multiplicity of a bond: Greater the multiplicity of the bonds less is the bond length. Like a compound with triple bond has less bond length, then the compound with double bonds, while a compound with single bonds has greatest bond length.
-Types of hybridization: S orbital has a small size, so, more is the s character, and less is the bond length.
- Delocalization of electrons through resonance also affects the size of bonds.
Since, the compound contains unsaturation, we will see the resonance factor. The resonating structures of the compound are,

We can see that C1-C2 are having a partial double bond character throughout the delocalization of electrons, while C3-C4 are having a single bond character.
As stated before, that multiplicity of bonds reduces the bond length, while the bond length of a single bond is more, so, due to the resonating structures, C1-C2 has less bond length than C3-C4.
Hence, option (b), C1-C2 bond is shorter than C3-C4 bond is correct.
Note: Bond order which is the number of bonds present also affects the length of bonds. More bond order means less bond length, this factor is also related to resonating structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




