
Which of the following processes will you use to convert aniline to benzyl amine?
A.$NaN{O_2} + HCl,CuCN,{H_2}/Ni$
B.$B{r_2}/CC{l_4},KCN,LiAl{H_4}$
C.$HN{O_2},{K_2}C{r_2}{O_7}/{H^ + },Sn + HCl$
D.$C{H_3}OH,KMn{O_4},O{H^ - },H_3^ + O$
Answer
582.9k+ views
Hint:Aniline is converted to benzene diazonium chloride at the temperature of $273 - 278K$ in the presence of a catalyst. Then, this obtained compound is converted to benzonitrile by displacing ${N_2}Cl$ by $CN$. Now, it is reduced to form benzyl amine.
Complete step by step answer:
Anilines are also called amino benzene or phenylamine. This compound has the formula as ${C_6}{H_5}N{H_2}$ in which the phenyl group is attached with an amino group. This compound is also soluble in water.
This organic compound when exposed to air and light becomes dark. It is a weak base and can react with strong acids. It is toxic. It can also be oxidized to form carbon – nitrogen bonds. Amides can also be formed when it reacts with carboxylic acid. When reacting with strong acids it can form ions of anilinium.
The structural formula for benzyl amine is ${C_6}{H_5}C{H_2}N{H_2}$. This consists of a benzyl group which is attached to an amine functional group. It is colourless and is also water soluble liquid which is used in the manufacture of many pharmaceuticals.
The processes to convert aniline to benzyl amine are:
-Benzene diazonium chloride is formed when aniline is treated with $NaN{O_2}$ and $HCl$, which is then reacted with $CuCN/KCN$ to replace ${N_2}Cl$ with $CN$, which is called as Sandmeyer’s Reaction and forms Benzonitrile.
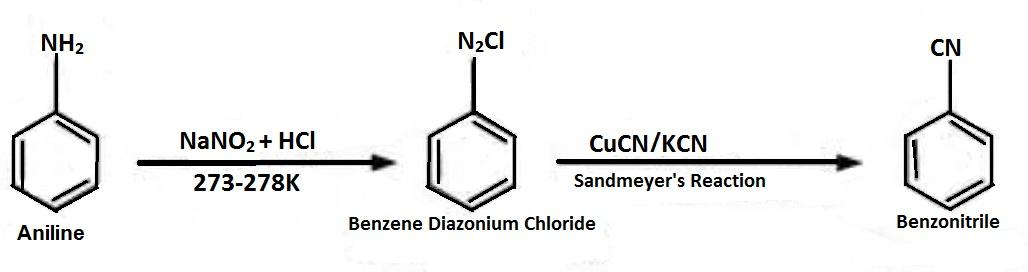
-Then the benzonitrile is reduced to form benzyl amine by using $LiAl{H_4}$ or lithium aluminium hydride.
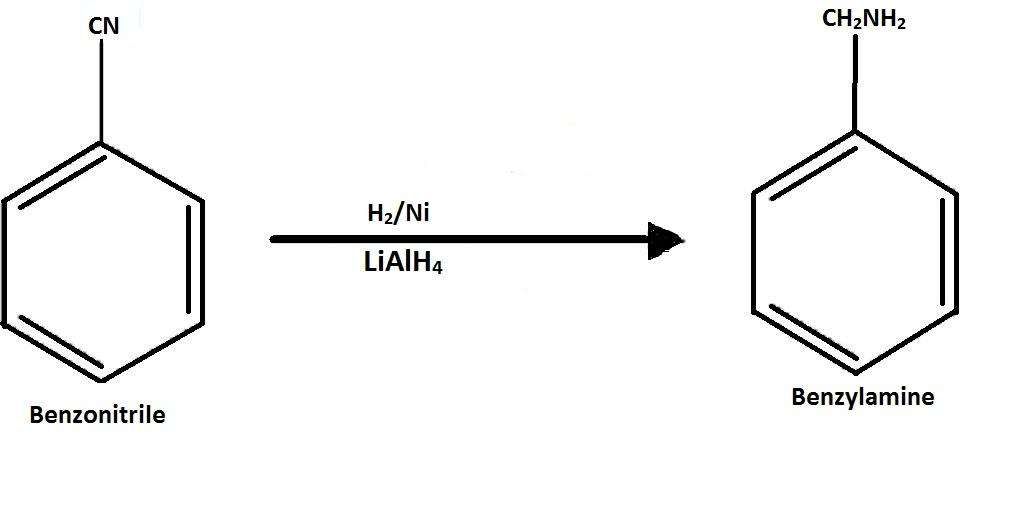
Hence, to convert the aniline to benzylamine the process used is $NaN{O_2} + HCl,CuCN,{H_2}/Ni$.
So, the correct answer is option A.
Note:Benzyl amine has a molar mass of $107.105g/mol$. It is colourless liquid with ammonia like odour.
The Sandmeyer’s reaction is the reaction which is used for the production of aryl halides from aryl diazonium salts using reagents or catalysts. This reaction can be used to carry out many transformations on benzene like halogenation, cyanation, hydroxylation and trifluoromethylation.
Complete step by step answer:
Anilines are also called amino benzene or phenylamine. This compound has the formula as ${C_6}{H_5}N{H_2}$ in which the phenyl group is attached with an amino group. This compound is also soluble in water.
This organic compound when exposed to air and light becomes dark. It is a weak base and can react with strong acids. It is toxic. It can also be oxidized to form carbon – nitrogen bonds. Amides can also be formed when it reacts with carboxylic acid. When reacting with strong acids it can form ions of anilinium.
The structural formula for benzyl amine is ${C_6}{H_5}C{H_2}N{H_2}$. This consists of a benzyl group which is attached to an amine functional group. It is colourless and is also water soluble liquid which is used in the manufacture of many pharmaceuticals.
The processes to convert aniline to benzyl amine are:
-Benzene diazonium chloride is formed when aniline is treated with $NaN{O_2}$ and $HCl$, which is then reacted with $CuCN/KCN$ to replace ${N_2}Cl$ with $CN$, which is called as Sandmeyer’s Reaction and forms Benzonitrile.

-Then the benzonitrile is reduced to form benzyl amine by using $LiAl{H_4}$ or lithium aluminium hydride.

Hence, to convert the aniline to benzylamine the process used is $NaN{O_2} + HCl,CuCN,{H_2}/Ni$.
So, the correct answer is option A.
Note:Benzyl amine has a molar mass of $107.105g/mol$. It is colourless liquid with ammonia like odour.
The Sandmeyer’s reaction is the reaction which is used for the production of aryl halides from aryl diazonium salts using reagents or catalysts. This reaction can be used to carry out many transformations on benzene like halogenation, cyanation, hydroxylation and trifluoromethylation.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




