
How is the total number of electrons represented in a Lewis structure determined.
Answer
552k+ views
Hint: The total number of electrons in the Lewis structure is the total number of valence electrons shared by each atom in the molecule. The valence electrons are the electrons present in the outermost electronic configuration of the chemical element.
Complete step by step answer:
The Lewis symbols also referred to as Lewis dot diagrams or the electron dot diagrams are the diagrams which show the valence electrons of an atom.
The Lewis structure also known as Lewis dot structure or electron dot structure are the diagrams which show the valence electrons of the atoms within a molecule.
The Lewis structure and the Lewis symbols helps to visualize the valence electrons of the atoms or the molecules whether they are present as non-bonding electrons (lone pairs) or bonding electrons (present within bond).
The total number of valence electrons represented in the Lewis structure can be determined by counting the valence electrons of the atom present in the molecule and adding the valence electrons.
The valence electrons are defined as the electrons present in the outermost electronic configuration of the chemical element. The valence electrons take part in the bonding process. In the Lewis structure, the valence electrons are represented as small dots.
Example: Drawing the Lewis structure of water ${H_2}O$.
In water two hydrogen atoms are present and one oxygen atom is present. The atomic number of hydrogen is 1 and the electronic configuration of hydrogen is $1{s^1}$. The valence electron of hydrogen is 1. The atomic number of oxygen is 8 and the electronic configuration of oxygen is $[He]2{s^2}2{p^4}$. The valence electrons of oxygen is 6.
Total number of valence electrons is $1 \times 2 + 6 = 8$.
The Lewis structure of ${H_2}O$ is shown below.
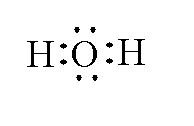
Note: In case of any ionic compound, to the total number of valence electrons given by the compound the charge of the compound is also added as electron gains electron to form anion and in case of cationic compound the charge of the compound is subtracted from the total valence electrons given by the compound as it loses electrons to form cation.
Complete step by step answer:
The Lewis symbols also referred to as Lewis dot diagrams or the electron dot diagrams are the diagrams which show the valence electrons of an atom.
The Lewis structure also known as Lewis dot structure or electron dot structure are the diagrams which show the valence electrons of the atoms within a molecule.
The Lewis structure and the Lewis symbols helps to visualize the valence electrons of the atoms or the molecules whether they are present as non-bonding electrons (lone pairs) or bonding electrons (present within bond).
The total number of valence electrons represented in the Lewis structure can be determined by counting the valence electrons of the atom present in the molecule and adding the valence electrons.
The valence electrons are defined as the electrons present in the outermost electronic configuration of the chemical element. The valence electrons take part in the bonding process. In the Lewis structure, the valence electrons are represented as small dots.
Example: Drawing the Lewis structure of water ${H_2}O$.
In water two hydrogen atoms are present and one oxygen atom is present. The atomic number of hydrogen is 1 and the electronic configuration of hydrogen is $1{s^1}$. The valence electron of hydrogen is 1. The atomic number of oxygen is 8 and the electronic configuration of oxygen is $[He]2{s^2}2{p^4}$. The valence electrons of oxygen is 6.
Total number of valence electrons is $1 \times 2 + 6 = 8$.
The Lewis structure of ${H_2}O$ is shown below.
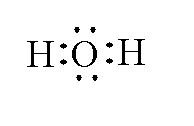
Note: In case of any ionic compound, to the total number of valence electrons given by the compound the charge of the compound is also added as electron gains electron to form anion and in case of cationic compound the charge of the compound is subtracted from the total valence electrons given by the compound as it loses electrons to form cation.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE





