
The value of Q10 for photosynthesis is
(a) 4
(b) 6
(c) 7
(d) 2
Answer
593.4k+ views
Hint: To solve this question, we have to have a clear concept on Q10 value of photosynthesis. First, we should know about some basics of photosynthesis. Try to remember the definition and the value.
Complete answer:The Q10 or the temperature coefficient is a measure of the
rate of change of a biological reaction with increment of the temperature by 10֯ C.
The value of the same for photosynthesis is 2.
Additional information:Photosynthesis is a biological process by which leaves of plants make their own food (glucose) in presence of sunlight and takes carbon dioxide and water from the environment in the chlorophyll and releases oxygen.
1. Respiration is a biological process that takes place in living organisms and involves the production of energy. During this process intake of oxygen and the release of carbon dioxide takes place from the oxidation of complex organic substances.
2. Photosynthesis occurs only in plants whereas respiration occurs both in animals and plants.
3. In the 19–29°C temperature range, average Q10 values are 1.9 for respiration and 2.4 for photosynthesis, meaning that respiration would almost double and photosynthesis is more than double with a temperature increase of 10 °C.
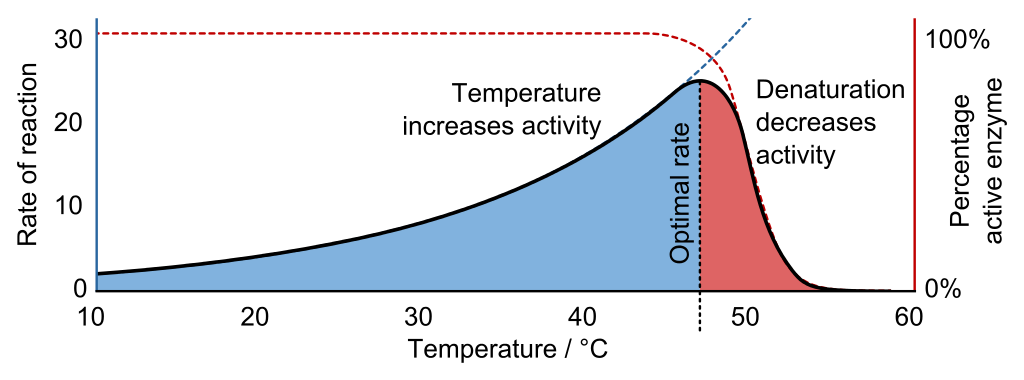
4. Up to a certain level of temperature increase, the rate of photosynthesis increases.
5. After that temperature, denaturation starts and the rate of photosynthesis decreases.
6. The temperature on which the rate of photosynthesis is highest is called the optimum temperature.
So, the final answer is option D. 2
Note:Remember the values of Q 10 For photosynthesis and respiration. This Q10 value helps to understand the relation between reaction rate and temperature. A bell shaped curve is seen where it is clearly seen that as the temperature increases by 100C, the rate of reaction also increases, but up to a certain level after that increment of temperature will not increase the rate but reaction, instead denaturation decreases the activity.
Complete answer:The Q10 or the temperature coefficient is a measure of the
rate of change of a biological reaction with increment of the temperature by 10֯ C.
The value of the same for photosynthesis is 2.
Additional information:Photosynthesis is a biological process by which leaves of plants make their own food (glucose) in presence of sunlight and takes carbon dioxide and water from the environment in the chlorophyll and releases oxygen.
1. Respiration is a biological process that takes place in living organisms and involves the production of energy. During this process intake of oxygen and the release of carbon dioxide takes place from the oxidation of complex organic substances.
2. Photosynthesis occurs only in plants whereas respiration occurs both in animals and plants.
3. In the 19–29°C temperature range, average Q10 values are 1.9 for respiration and 2.4 for photosynthesis, meaning that respiration would almost double and photosynthesis is more than double with a temperature increase of 10 °C.

Fig: Q10 graph
4. Up to a certain level of temperature increase, the rate of photosynthesis increases.
5. After that temperature, denaturation starts and the rate of photosynthesis decreases.
6. The temperature on which the rate of photosynthesis is highest is called the optimum temperature.
So, the final answer is option D. 2
Note:Remember the values of Q 10 For photosynthesis and respiration. This Q10 value helps to understand the relation between reaction rate and temperature. A bell shaped curve is seen where it is clearly seen that as the temperature increases by 100C, the rate of reaction also increases, but up to a certain level after that increment of temperature will not increase the rate but reaction, instead denaturation decreases the activity.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




