
The structure of the compound with molecular formula ${ C }_{ 6 }{ H }_{ 12 }$ that has only secondary H-atom is
A) ${ 1-methyl cyclopentane }$
B) ${ 1,1-Dimethyl cyclobutane }$
C) Cyclohexane
D) ${ 2-Methyl pent-2-ene }$
Answer
600k+ views
Hint: A primary hydrogen is a hydrogen atom residing on a primary carbon in an organic species and secondary hydrogen is a hydrogen atom on a carbon attached to another two carbons.
Complete step-by-step answer:
A)
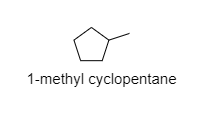
In this compound, the methyl groups have primary hydrogens that are also present along with secondary hydrogens.
B)
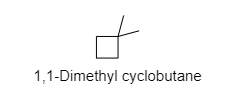
In this compound also, the methyl groups have primary hydrogens that are also present along with secondary hydrogens.
C)
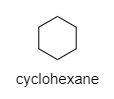
In this compound, a carbon atom is attached to another two carbons, so it has all the secondary hydrogens present.
D)
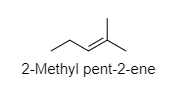
In ${ 2-Methyl pent-2-ene }$, the methyl groups have primary hydrogens that are also present along with secondary hydrogens.
Hence, The structure of the compound with molecular formula ${ C }_{ 6 }{ H }_{ 12 }$ the only secondary H-atom is cyclohexane.
The correct option is C.
Additional Information:
Structural isomerism, or constitutional isomerism (per IUPAC), is a form of isomerism in which molecules with the same molecular formula have bonded together in different orders, as opposed to stereoisomerism. There are multiple synonyms for constitutional isomers.
Three categories of constitutional isomers are skeletal, positional, and functional isomers. Positional isomers are also called regioisomers. Tautomers are a subcategory of functional isomers.
Note: The possibility to make a mistake is that you may choose option A. But in ${ 1-methyl cyclopentane }$, the cyclopentane has all the secondary hydrogens but the methyl group attached to it has primary hydrogens also.
Complete step-by-step answer:
A)

In this compound, the methyl groups have primary hydrogens that are also present along with secondary hydrogens.
B)

In this compound also, the methyl groups have primary hydrogens that are also present along with secondary hydrogens.
C)

In this compound, a carbon atom is attached to another two carbons, so it has all the secondary hydrogens present.
D)

In ${ 2-Methyl pent-2-ene }$, the methyl groups have primary hydrogens that are also present along with secondary hydrogens.
Hence, The structure of the compound with molecular formula ${ C }_{ 6 }{ H }_{ 12 }$ the only secondary H-atom is cyclohexane.
The correct option is C.
Additional Information:
Structural isomerism, or constitutional isomerism (per IUPAC), is a form of isomerism in which molecules with the same molecular formula have bonded together in different orders, as opposed to stereoisomerism. There are multiple synonyms for constitutional isomers.
Three categories of constitutional isomers are skeletal, positional, and functional isomers. Positional isomers are also called regioisomers. Tautomers are a subcategory of functional isomers.
Note: The possibility to make a mistake is that you may choose option A. But in ${ 1-methyl cyclopentane }$, the cyclopentane has all the secondary hydrogens but the methyl group attached to it has primary hydrogens also.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




