
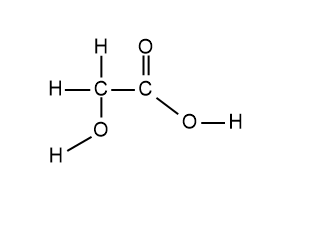
The structure of glycolic acid is shown below.
Long-chain alkanes can be cracked to produce shorter chain alkanes and alkanes.
i.What conditions are needed for cracking?
ii.Complete the equation for the cracking of hexadecane,\[{C_{16}}{H_{24}}\], form octane, \[{C_8}{H_{18}}\]and ethene only.
\[{C_{16}}{H_{24}} \to {\text{ }}{C_8}{H_{18}} + \ldots \ldots \ldots {C_2}{H_4}\]

Answer
514.5k+ views
Hint: Cracking is used in petroleum refining. It is the most important process for the production of gasoline and diesel fuel commercially. Cracking of petroleum different types of oils like light oils, middle-range oils, and heavy oils. The first thermal cracking was done in $1913$.
Complete answer: Cracking is defined as a process, wherein the long-chain hydrocarbons are broken down into light hydrocarbons. The long-chain hydrocarbons are complex organic molecules that need to be broken by breaking the bonds between the carbon-carbon atoms. The process of cracking is used in the industries for the production of petrol, diesel, and gasoline. There are certain conditions under which the cracking process takes place and the conditions are high temperature (usually in the range of ${450^0}$ to ${750^0}$) and pressures (up to ${70^0}$ atmospheres) and sometimes the presence of a catalyst. There are several types of cracking like thermal, steam, hydro and fluid catalytic cracking
Hexadecane \[{C_{16}}{H_{24}}\] is a complex long chain hydrocarbon. On undergoing the process of cracking, hexadecane will yield octane and ethene. The complete reaction for this is:
\[{C_{16}}{H_{24}} \to {\text{ }}{C_8}{H_{18}} + 4{C_2}{H_4}\]
Alkenes are produced as a result of thermal cracking.
Note:
When cracking takes place the complex hydrocarbons are broken down into smaller hydrocarbons, this way the fuel supply and the production are increased. This helps in balancing the demand with the supply of fuels. This is the only process that can break down the larger hydrocarbons into smaller ones.
Complete answer: Cracking is defined as a process, wherein the long-chain hydrocarbons are broken down into light hydrocarbons. The long-chain hydrocarbons are complex organic molecules that need to be broken by breaking the bonds between the carbon-carbon atoms. The process of cracking is used in the industries for the production of petrol, diesel, and gasoline. There are certain conditions under which the cracking process takes place and the conditions are high temperature (usually in the range of ${450^0}$ to ${750^0}$) and pressures (up to ${70^0}$ atmospheres) and sometimes the presence of a catalyst. There are several types of cracking like thermal, steam, hydro and fluid catalytic cracking
Hexadecane \[{C_{16}}{H_{24}}\] is a complex long chain hydrocarbon. On undergoing the process of cracking, hexadecane will yield octane and ethene. The complete reaction for this is:
\[{C_{16}}{H_{24}} \to {\text{ }}{C_8}{H_{18}} + 4{C_2}{H_4}\]
Alkenes are produced as a result of thermal cracking.
Note:
When cracking takes place the complex hydrocarbons are broken down into smaller hydrocarbons, this way the fuel supply and the production are increased. This helps in balancing the demand with the supply of fuels. This is the only process that can break down the larger hydrocarbons into smaller ones.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




