
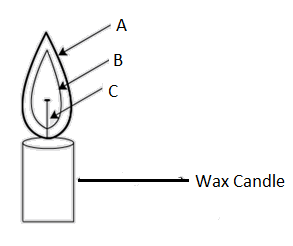
The different zones of a candle flame are marked by the letters A, B and C. Which of the following is correct?
A. B is the hottest part of the flame
B. C is moderately hot
C. A is the hottest part of the flame
D. A is moderately hot whereas C is the coldest part

Answer
582.9k+ views
Hint: A prior knowledge of combustion reaction is necessary to understand the different phases of a flame.
Combustion reactions are the reactions in which fuel reacts with oxygen and releases a huge amount of energy generally in the form of light and heat. Atoms of the reactants get superheated by the virtue of the heat dissipated and as these atoms leave the combustion zone, they give out the excess energy in the form of light which we see as a flame.
Complete step by step answer:
Flames are generally nothing but the visible part of a combustion reaction and are also divided into three different zones based on the extent of availability of oxygen, each distinguishable by their distinct color (which are labeled as A, B and C)
C is the innermost zone closest to the wick and is also known as the dark zone as it doesn’t support burning and contains the unburnt particles of the carbon due to the lack of supply of air (oxygen).
B is the biggest part of the flame distinguished by different shades of orange and yellow. It is also the luminous zone of the flame as it emits and the zone where incomplete combustion takes place due to moderate supply of air.
A , the outermost part of the flame gets an unlimited supply of oxygen and thus witnesses complete combustion with the excessive energy being dissipated more as heat energy than light distinguishable by a characteristic blue color.
So, the correct answer is “Option C”.
Note: One must remember that along with C as the Dark zone, A and B are also referred as Non-Luminous and Luminous zones respectively based on their ability to produce and emit light.
Combustion reactions are the reactions in which fuel reacts with oxygen and releases a huge amount of energy generally in the form of light and heat. Atoms of the reactants get superheated by the virtue of the heat dissipated and as these atoms leave the combustion zone, they give out the excess energy in the form of light which we see as a flame.
Complete step by step answer:
Flames are generally nothing but the visible part of a combustion reaction and are also divided into three different zones based on the extent of availability of oxygen, each distinguishable by their distinct color (which are labeled as A, B and C)
C is the innermost zone closest to the wick and is also known as the dark zone as it doesn’t support burning and contains the unburnt particles of the carbon due to the lack of supply of air (oxygen).
B is the biggest part of the flame distinguished by different shades of orange and yellow. It is also the luminous zone of the flame as it emits and the zone where incomplete combustion takes place due to moderate supply of air.
A , the outermost part of the flame gets an unlimited supply of oxygen and thus witnesses complete combustion with the excessive energy being dissipated more as heat energy than light distinguishable by a characteristic blue color.
So, the correct answer is “Option C”.
Note: One must remember that along with C as the Dark zone, A and B are also referred as Non-Luminous and Luminous zones respectively based on their ability to produce and emit light.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




