
The correct statement (s) for the following addition reactions is (are):
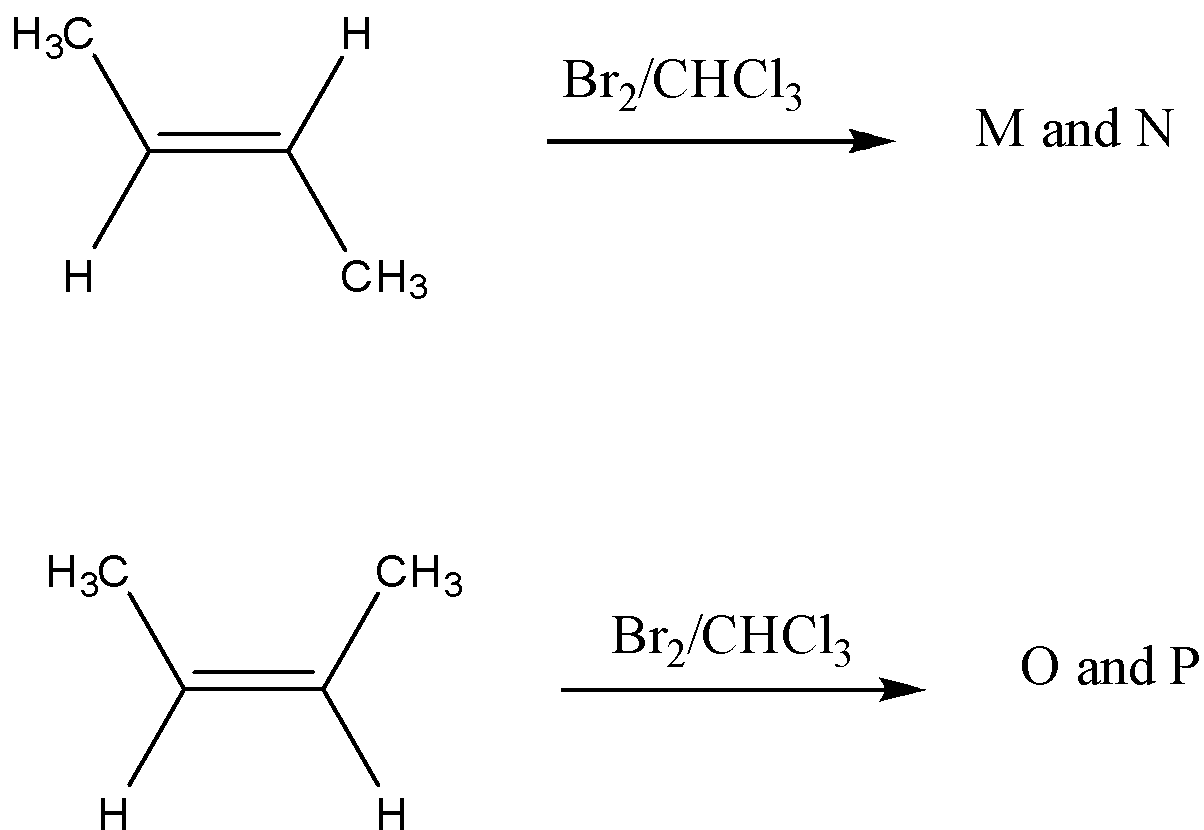
This question has multiple correct options
A.Bromination proceeds through trans-addition in both the reactions.
B.O and P are identical molecules.
C.(M and O) and (N and P) are two pairs of diastereomers.
D.(M and O) and (N and P) are two pairs of enantiomers.

Answer
577.5k+ views
Hint:The given compounds are trans-2-butene and cis-2-butene. The given reactions are examples of stereospecific reactions. We can say that products obtained could be meso products, racemic mixtures. Draw the representation of the product to identify the correct statements.
Complete step by step answer:
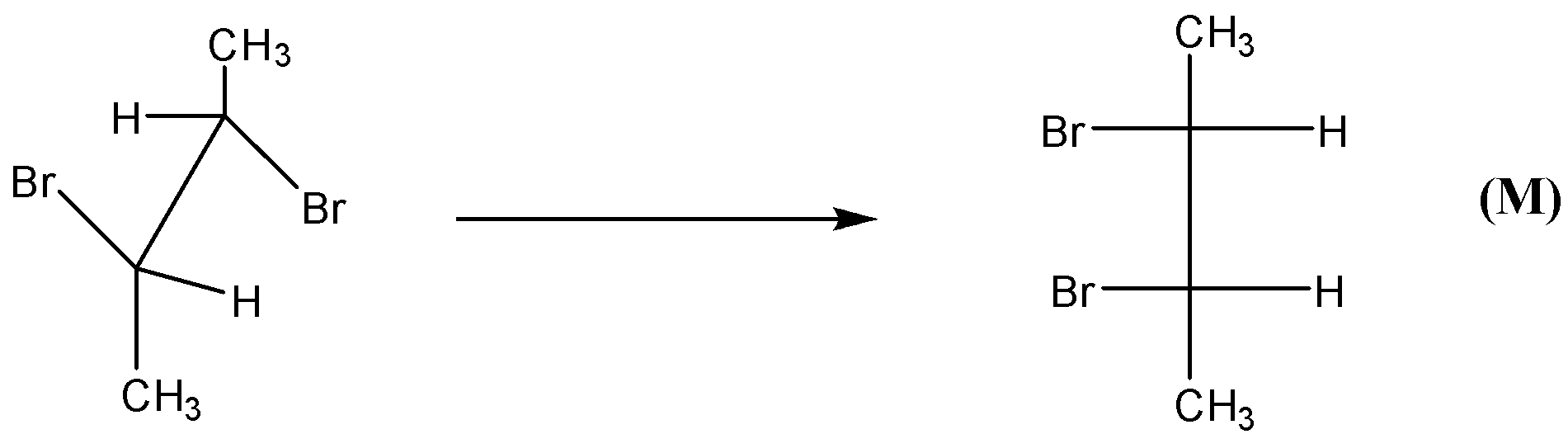
-Now, in this question we know that there is addition of halogen bromine to trans-2-butene and cis-2-butene. As mentioned these are examples of stereospecific reactions.
-We have shown in the figure the formation of the product. M and N products are formed from the reaction of trans-2-butene.
-We can clearly see that trans-2-butene provides meso products. We can say that M and N obtained are identical molecules.
-Now, if we see the given options, then the first is trans-addition in both the reactions.
-Thus, from the figure we can say that bromination occurs through trans-addition.
-Second, we have O and P are identical molecules. But from the figure we can see that O and P are not identical.
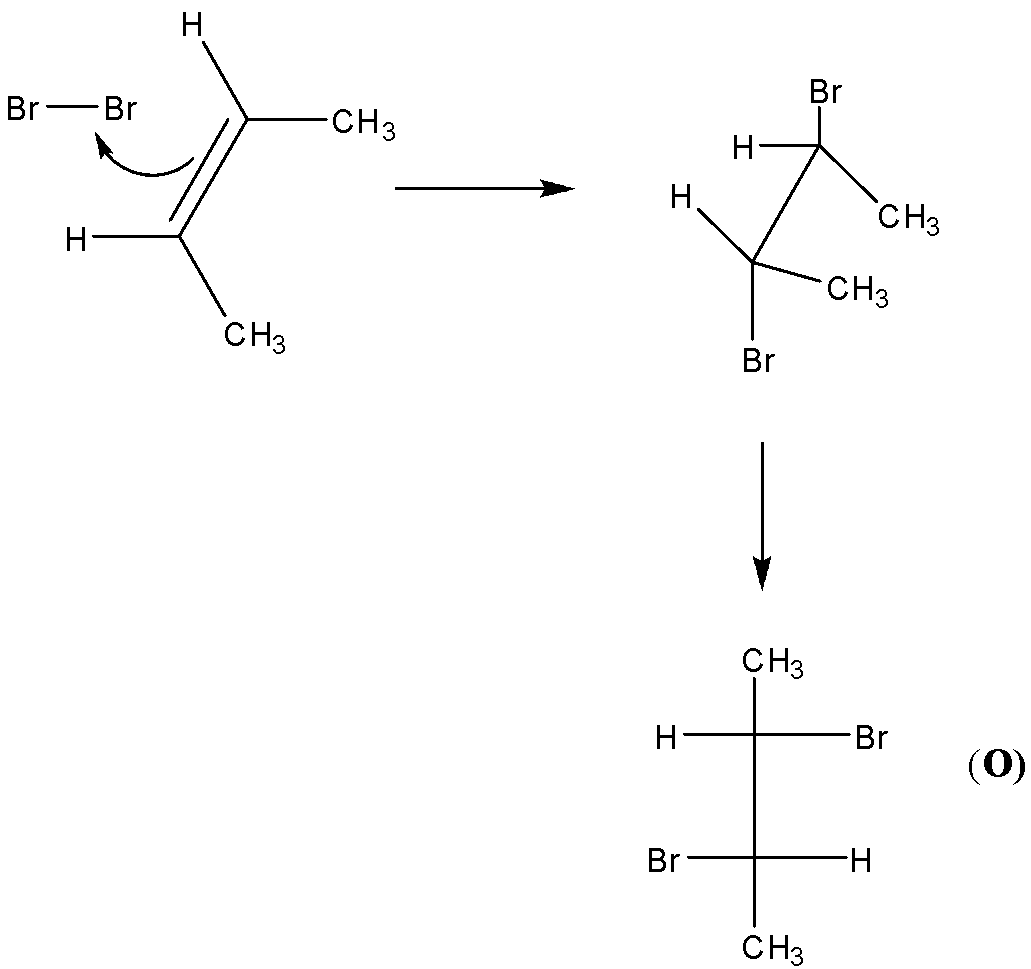
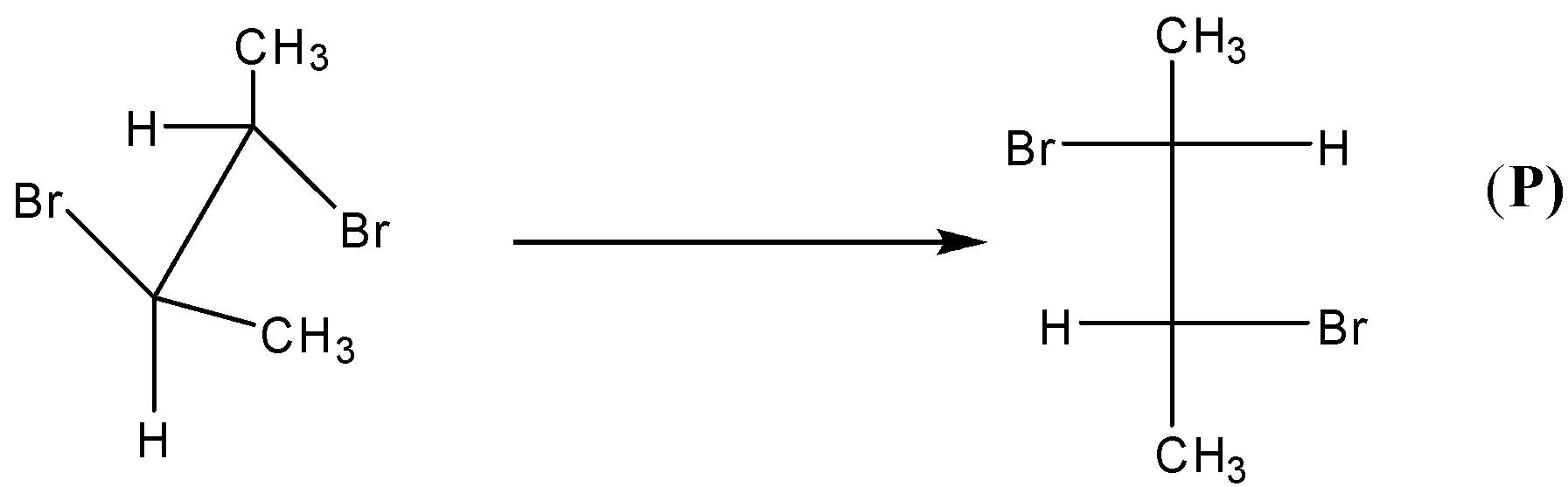
-We know that cis-2-butene forms racemic mixture on bromination. So, we can say that O and P are pair of enantiomers.
-Thus, O and P are considered to be non-superimposable images of each other.
-Third, we have to identify that M and O, N and P represents pairs of diastereomers.
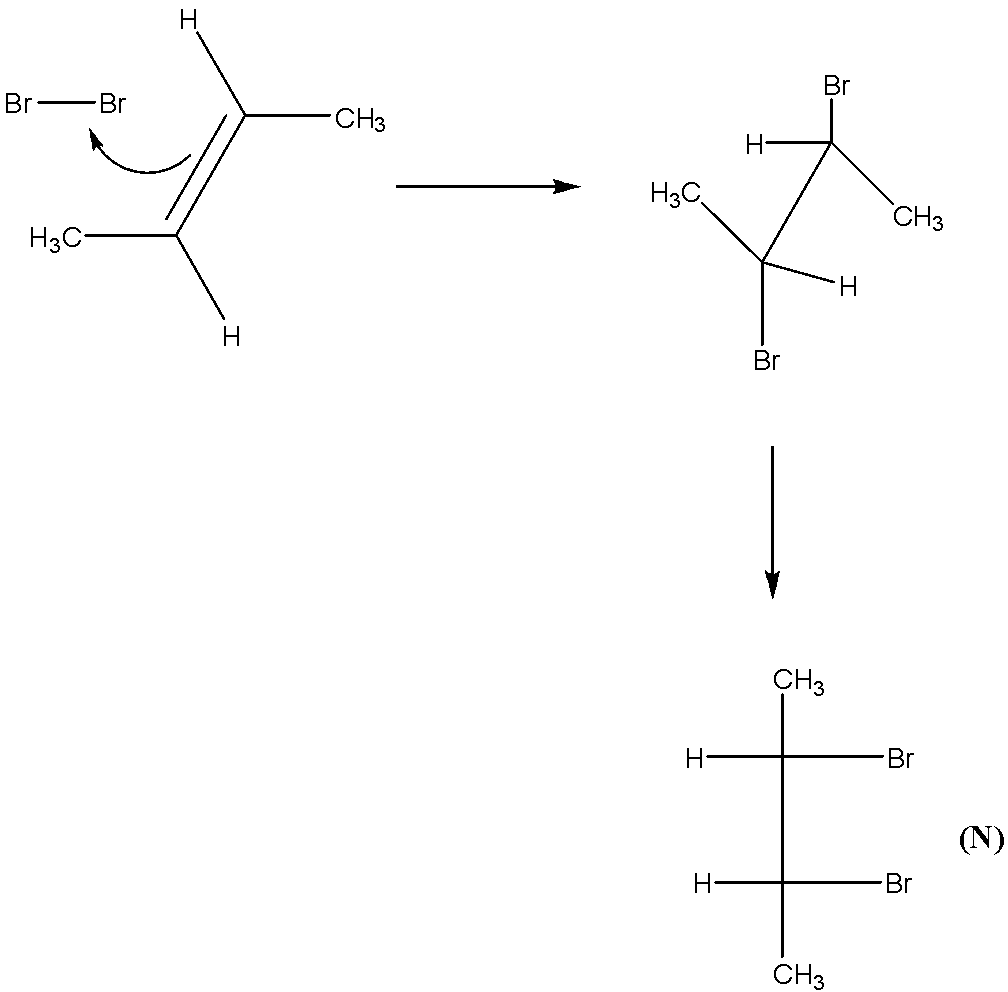
-We can see that they exhibit the same configuration at the chiral atom, and different at another one.
-So, we can say that these pairs are considered to be pairs of diastereomers.
-Now, the fourth we have M and O, N and P are pairs of enantiomers.
-As mentioned above, we know that O and P are enantiomers, and M and N, O and P are pairs of diastereomers.
-In the last we can conclude that the trans-bromonium ion is formed, and (M and O) and (N and P) are pairs of diastereomers.
Hence, the correct options are A and C.
Note:
As we have used the terms like diastereomers, enantiomers, racemic mixture and meso product. These terms can be defined as:
-Diastereomers: It is a type of stereoisomers when there are more than two stereocenters in the structure. It is also defined as non-mirror images and non-superimposable images of one another.
-Enantiomers: It is defined as chiral molecules which are mirror images to each other but non-superimposable on one another.
-Racemic mixture: In the reaction, there are two enantiomers obtained in equal proportions, i.e. 50:50 and have zero optical rotation.
-Meso product: It has a superimposable image on its mirror image. These are achiral compounds, i.e. has multiple chiral centres in the product.
Complete step by step answer:
-Now, in this question we know that there is addition of halogen bromine to trans-2-butene and cis-2-butene. As mentioned these are examples of stereospecific reactions.
-We have shown in the figure the formation of the product. M and N products are formed from the reaction of trans-2-butene.
-We can clearly see that trans-2-butene provides meso products. We can say that M and N obtained are identical molecules.
-Now, if we see the given options, then the first is trans-addition in both the reactions.
-Thus, from the figure we can say that bromination occurs through trans-addition.
-Second, we have O and P are identical molecules. But from the figure we can see that O and P are not identical.


-We know that cis-2-butene forms racemic mixture on bromination. So, we can say that O and P are pair of enantiomers.
-Thus, O and P are considered to be non-superimposable images of each other.
-Third, we have to identify that M and O, N and P represents pairs of diastereomers.


-We can see that they exhibit the same configuration at the chiral atom, and different at another one.
-So, we can say that these pairs are considered to be pairs of diastereomers.
-Now, the fourth we have M and O, N and P are pairs of enantiomers.
-As mentioned above, we know that O and P are enantiomers, and M and N, O and P are pairs of diastereomers.
-In the last we can conclude that the trans-bromonium ion is formed, and (M and O) and (N and P) are pairs of diastereomers.
Hence, the correct options are A and C.
Note:
As we have used the terms like diastereomers, enantiomers, racemic mixture and meso product. These terms can be defined as:
-Diastereomers: It is a type of stereoisomers when there are more than two stereocenters in the structure. It is also defined as non-mirror images and non-superimposable images of one another.
-Enantiomers: It is defined as chiral molecules which are mirror images to each other but non-superimposable on one another.
-Racemic mixture: In the reaction, there are two enantiomers obtained in equal proportions, i.e. 50:50 and have zero optical rotation.
-Meso product: It has a superimposable image on its mirror image. These are achiral compounds, i.e. has multiple chiral centres in the product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




