
The letters D and L in carbohydrates represents:
A.Optical rotation
B.Mutarotation
C.Direct synthesis
D.Configuration
Answer
587.4k+ views
Hint: The letters D and L represent the position of the second last hydroxyl group in a carbohydrate.
-If the carbohydrate has the hydroxyl group at the last chiral carbon towards the right side, it is denoted by the letter D and if the hydroxyl group at the last chiral carbon lies towards the left side, it is denoted by the letter L.
Complete step by step answer:
Let us take the example of a carbohydrate, glucose. The correct name of glucose is D-(+)-Glucose. Since glucose is optically active, its enantiomer is named as L-(+)-Glucose.
The letters D and L used before the name of glucose represent the configuration of the hydroxyl group at the penultimate carbon atom, i.e. at the last but one carbon atom of the carbon chain.
On the other hand, the algebraic signs ‘+’ and ‘-‘ within brackets after the letters D and L represent the signs of optical rotation. Or in other words, they refer to dextrorotatory and laevorotatory respectively.
The letters D and L have no relation with the sign of optical rotation. This means a carbohydrate having ‘D’ configuration may be either dextrorotatory or laevorotatory and a carbohydrate having ‘L’ configuration may also be either dextrorotatory or laevorotatory. For example, D- glucose is dextrorotatory while D- fructose is laevorotatory.
The meaning of D and L configurations can be explained with the help of glyceraldehyde. Glyceraldehyde is chosen as the standard for assigning D and L configurations to monosaccharides. It is the simplest carbohydrate as it contains one chiral carbon and hence exists in two enantiomeric forms.
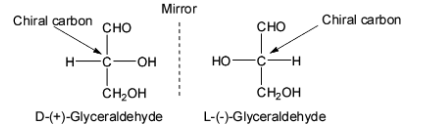
Thus, all the monosaccharides which are chemically related to the ‘+’ isomer of glyceraldehyde are assigned the D configuration and those correlated to the ‘-‘ isomer of glyceraldehyde are assigned the L configuration.
So, the correct option is D.
Note:
For assigning these configurations, the structures of the monosaccharides are written in such a way that the most oxidized carbon, i.e. the aldehyde group is at the top.
Thus, according to this discussion, (+) glucose is assigned D configuration and (-) glucose is assigned the L configuration.

-If the carbohydrate has the hydroxyl group at the last chiral carbon towards the right side, it is denoted by the letter D and if the hydroxyl group at the last chiral carbon lies towards the left side, it is denoted by the letter L.
Complete step by step answer:
Let us take the example of a carbohydrate, glucose. The correct name of glucose is D-(+)-Glucose. Since glucose is optically active, its enantiomer is named as L-(+)-Glucose.
The letters D and L used before the name of glucose represent the configuration of the hydroxyl group at the penultimate carbon atom, i.e. at the last but one carbon atom of the carbon chain.
On the other hand, the algebraic signs ‘+’ and ‘-‘ within brackets after the letters D and L represent the signs of optical rotation. Or in other words, they refer to dextrorotatory and laevorotatory respectively.
The letters D and L have no relation with the sign of optical rotation. This means a carbohydrate having ‘D’ configuration may be either dextrorotatory or laevorotatory and a carbohydrate having ‘L’ configuration may also be either dextrorotatory or laevorotatory. For example, D- glucose is dextrorotatory while D- fructose is laevorotatory.
The meaning of D and L configurations can be explained with the help of glyceraldehyde. Glyceraldehyde is chosen as the standard for assigning D and L configurations to monosaccharides. It is the simplest carbohydrate as it contains one chiral carbon and hence exists in two enantiomeric forms.

Thus, all the monosaccharides which are chemically related to the ‘+’ isomer of glyceraldehyde are assigned the D configuration and those correlated to the ‘-‘ isomer of glyceraldehyde are assigned the L configuration.
So, the correct option is D.
Note:
For assigning these configurations, the structures of the monosaccharides are written in such a way that the most oxidized carbon, i.e. the aldehyde group is at the top.
Thus, according to this discussion, (+) glucose is assigned D configuration and (-) glucose is assigned the L configuration.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




