
Anisole can be prepared by the action of methyl iodide on sodium phenate. The reaction is called:
(a)- Wurtz reaction
(b)- Williamson’s reaction
(c)- Fittig’s reaction
(d)- Etard reaction
Answer
590.4k+ views
Hint: Anisole is a chemical compound having ether as a functional group. In anisole, the benzene group is attached to the methyl group with an oxygen atom in between. When methyl iodide and sodium phenoxide is reacted, sodium iodide is the by-product.
Complete step by step answer:
Anisole is prepared by the action of methyl iodide on sodium phenoxide. This reaction is called Williamson’s synthesis.
Williamson's synthesis: This is one of the best methods for the preparation of ethers. It involves the treatment of an alkyl halide with a suitable sodium alkoxide. The sodium alkoxide needed for the purpose is prepared by the action of sodium on suitable alcohol. The reaction involves the nucleophilic displacement (substitution) of the halide ion from the alkyl halide by the alkoxide ion by the ${{S}_{N}}2$ mechanism. Thus, the general reaction of Williamson’s synthesis is given below.
\[2{{R}^{'}}-OH+2Na\to 2{{R}^{'}}-{{O}^{-}}N{{a}^{+}}+{{H}_{2}}\]
\[{{R}^{'}}-{{O}^{-}}N{{a}^{+}}+R-X\to {{R}^{'}}-O-R+NaX\]
This method can be used for the preparation of both symmetrical and unsymmetrical ethers.
Similarly, alkyl aryl ethers (phenolic ethers) can be easily prepared by treating sodium phenoxide with a suitable alkyl halide.
So the reaction of sodium phenoxide with methyl iodide is given below:
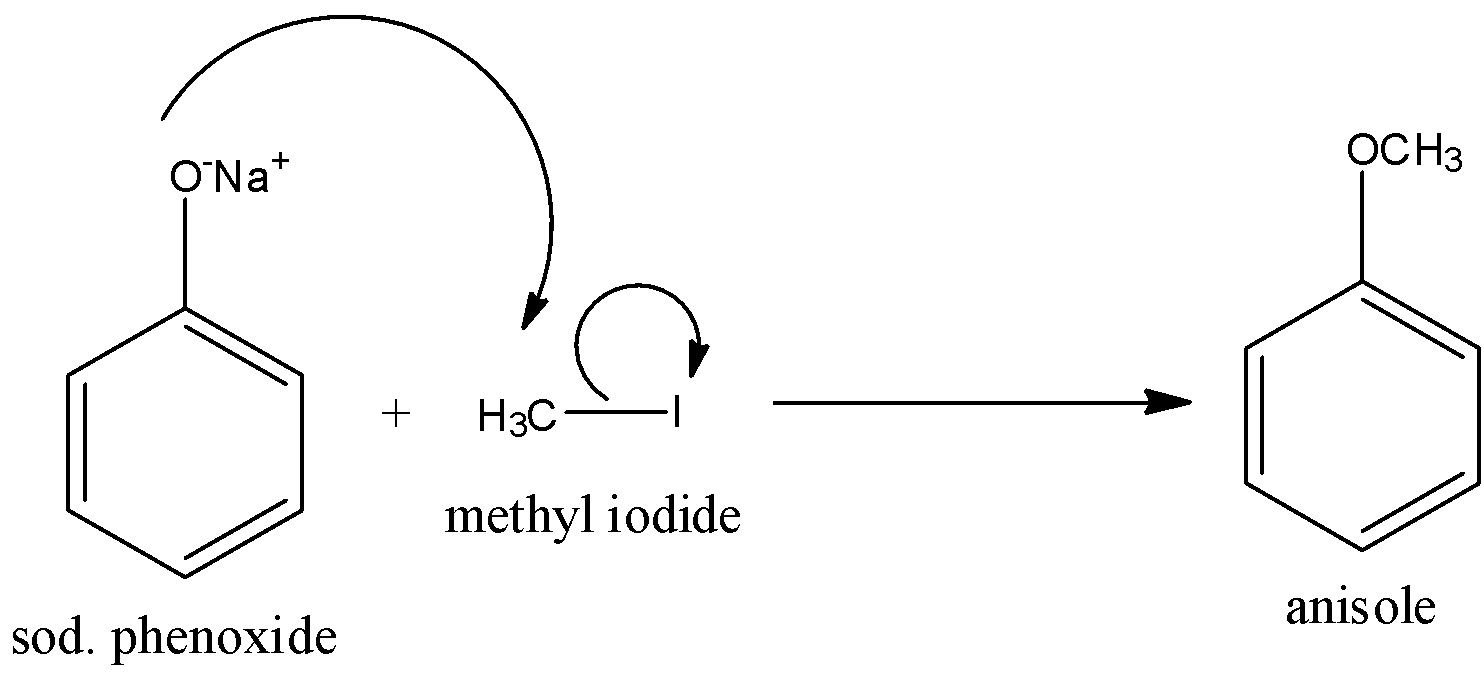
Hence, the correct answer is an option (b)- Williamson’s reaction.
Note: However ethers cannot be prepared by treating bromobenzene or iodobenzene with sodium salts of the corresponding alcohols, i.e., sodium methoxide, sodium ethoxide, or sodium allyl alkoxide. This is due to the reason that aryl halides are less reactive than the allyl halides towards nucleophilic substitution reaction.
Complete step by step answer:
Anisole is prepared by the action of methyl iodide on sodium phenoxide. This reaction is called Williamson’s synthesis.
Williamson's synthesis: This is one of the best methods for the preparation of ethers. It involves the treatment of an alkyl halide with a suitable sodium alkoxide. The sodium alkoxide needed for the purpose is prepared by the action of sodium on suitable alcohol. The reaction involves the nucleophilic displacement (substitution) of the halide ion from the alkyl halide by the alkoxide ion by the ${{S}_{N}}2$ mechanism. Thus, the general reaction of Williamson’s synthesis is given below.
\[2{{R}^{'}}-OH+2Na\to 2{{R}^{'}}-{{O}^{-}}N{{a}^{+}}+{{H}_{2}}\]
\[{{R}^{'}}-{{O}^{-}}N{{a}^{+}}+R-X\to {{R}^{'}}-O-R+NaX\]
This method can be used for the preparation of both symmetrical and unsymmetrical ethers.
Similarly, alkyl aryl ethers (phenolic ethers) can be easily prepared by treating sodium phenoxide with a suitable alkyl halide.
So the reaction of sodium phenoxide with methyl iodide is given below:

Hence, the correct answer is an option (b)- Williamson’s reaction.
Note: However ethers cannot be prepared by treating bromobenzene or iodobenzene with sodium salts of the corresponding alcohols, i.e., sodium methoxide, sodium ethoxide, or sodium allyl alkoxide. This is due to the reason that aryl halides are less reactive than the allyl halides towards nucleophilic substitution reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




