
The correct formula for nitrogen dioxide is:
A. \[NO\]
B.\[{N_2}O\]
C.\[N{O_2}\]
D.\[{N_2}{O_5}\]
Answer
581.7k+ views
Hint: To get to the answer we need to know the names of all the compounds in the options. They are Nitric oxide, Dinitrogen monoxide, Nitrogen dioxide and Dinitrogen pentoxide.
Step by step answer:
The molecular formula of nitrogen is \[N{O_2}\] . Here, \[{N_2}O\] stands for Dinitrogen monoxide. \[NO\] stands for Nitric oxide and \[{N_2}{O_5}\] stands for Dinitrogen pentoxide.
Here, nitrogen means one molecule of nitrogen and dioxide can be broken down as “di” meaning two and oxide meaning oxygen molecules i.e. two molecules of oxygen.
Therefore, answer is option C.
Additional information: Nitrogen dioxide is a chemical compound. It is one of the many nitrogen oxides. It is an extremely poisonous gas. It is a pollutant which absorbs UV light and does not allow it to reach to the earth’s surface. The structure of \[N{O_2}\] is as given in the diagram.
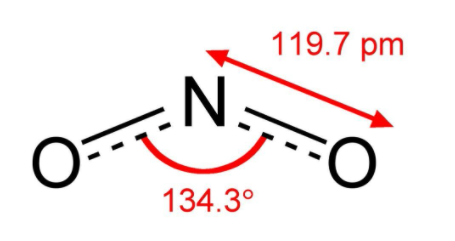
Nitrogen dioxide is a reddish brown gas above \[{212^ \circ }C\] with a pungent, acrid odour and becomes a yellowish brown liquid below \[{212^ \circ }C\] and converts to the colourless dinitrogen tetroxide below \[ - {11.2^ \circ }C\] . The bond length between the nitrogen atom and the oxygen atom is \[119.7pm\] and the angle formed is \[{134.3^ \circ }\].
Note: You need to know the molecular formula of all the compounds that you study. Having knowledge can help you solve such direct questions in seconds.
Step by step answer:
The molecular formula of nitrogen is \[N{O_2}\] . Here, \[{N_2}O\] stands for Dinitrogen monoxide. \[NO\] stands for Nitric oxide and \[{N_2}{O_5}\] stands for Dinitrogen pentoxide.
Here, nitrogen means one molecule of nitrogen and dioxide can be broken down as “di” meaning two and oxide meaning oxygen molecules i.e. two molecules of oxygen.
Therefore, answer is option C.
Additional information: Nitrogen dioxide is a chemical compound. It is one of the many nitrogen oxides. It is an extremely poisonous gas. It is a pollutant which absorbs UV light and does not allow it to reach to the earth’s surface. The structure of \[N{O_2}\] is as given in the diagram.

Nitrogen dioxide is a reddish brown gas above \[{212^ \circ }C\] with a pungent, acrid odour and becomes a yellowish brown liquid below \[{212^ \circ }C\] and converts to the colourless dinitrogen tetroxide below \[ - {11.2^ \circ }C\] . The bond length between the nitrogen atom and the oxygen atom is \[119.7pm\] and the angle formed is \[{134.3^ \circ }\].
Note: You need to know the molecular formula of all the compounds that you study. Having knowledge can help you solve such direct questions in seconds.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




