
The common basic structural unit in silicates and silica is-
A. $Si2O{6^{4 - }}$
B. $SiO{3^{2 - }}$
C.$SiO{4^{4 - }}$
D.$Si2O{7^{6 - }}$
Answer
591.3k+ views
Hint: Silicate is used to refer to the salts of silica, while silica is the silicon dioxide. Silicon is the second most abundant element in the earth's crust. It makes up about 27% of the average rock. Silicon binds up with oxygen (which makes up 55% of the earth's crust) to form the most common class of minerals, called the silicates.
Complete step by step answer:
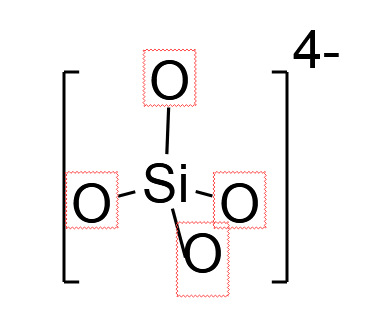
Silicate belongs to family of anions, having silicon and oxygen with the general formula ${\left[ {SiO_{4 - x}^{(4 - 2x) - }} \right]_n}$ where $0 \leqslant x < 2$ . The other members belonging to this family are orthosilicates $SiO_4^{4 - }$ (Here,$x = 0$), metasilicate $SiO_3^{2 - }$ (Here,$x = 1$), pyrosilicate $S{i_2}O_7^{6 - }$ (Here, $x = 0.5,n = 2$). The basic structural unit of any species is referred to the building block of that species. This means, if we keep on repeating that basic structural unit, we will get a long polymer chain of that particular species.
So, for the above asked question, we can clearly state that $SiO_4^{4 - }$ will be the basic structural unit in silicates. These anions are also called silicon tetroxide anion. Structurally, this anion is tetrahedral in shape, in which one silicon ($Si$) atom is surrounded by four oxygen ($O$) atoms.
Silica is another name for silicon dioxide, having the chemical formula $SiO_2$ . It is popularly found in nature in the form of quartz. Quartz is a silicate, composed of pure silica solid of silica. Quartz is the only polymorph of silica that is stable at the Earth's surface. Earth’s crust is composed of 59% silica.
Molecular $SiO_2$ has a linear structure. It is produced when molecular $SiO$ (silicon monoxide) is condensed in an $Ar$ (argon) matrix. The matrix is cooled with $He$ (helium) along with oxygen atoms, which are generated by microwave discharge.
So, the correct option is C.
Note:
-Finely divided crystals of silica are toxic in nature. It can lead to severe inflammation of the tissues present in lungs and thus can cause diseases such as lung cancer, silicosis and bronchitis.
-Silica finds its use in grinding and polishing stones and glasses, in manufacturing industries. It also finds its use as a gemstone.
Complete step by step answer:
Silicate belongs to family of anions, having silicon and oxygen with the general formula ${\left[ {SiO_{4 - x}^{(4 - 2x) - }} \right]_n}$ where $0 \leqslant x < 2$ . The other members belonging to this family are orthosilicates $SiO_4^{4 - }$ (Here,$x = 0$), metasilicate $SiO_3^{2 - }$ (Here,$x = 1$), pyrosilicate $S{i_2}O_7^{6 - }$ (Here, $x = 0.5,n = 2$). The basic structural unit of any species is referred to the building block of that species. This means, if we keep on repeating that basic structural unit, we will get a long polymer chain of that particular species.
So, for the above asked question, we can clearly state that $SiO_4^{4 - }$ will be the basic structural unit in silicates. These anions are also called silicon tetroxide anion. Structurally, this anion is tetrahedral in shape, in which one silicon ($Si$) atom is surrounded by four oxygen ($O$) atoms.

Silica is another name for silicon dioxide, having the chemical formula $SiO_2$ . It is popularly found in nature in the form of quartz. Quartz is a silicate, composed of pure silica solid of silica. Quartz is the only polymorph of silica that is stable at the Earth's surface. Earth’s crust is composed of 59% silica.
Molecular $SiO_2$ has a linear structure. It is produced when molecular $SiO$ (silicon monoxide) is condensed in an $Ar$ (argon) matrix. The matrix is cooled with $He$ (helium) along with oxygen atoms, which are generated by microwave discharge.
So, the correct option is C.
Note:
-Finely divided crystals of silica are toxic in nature. It can lead to severe inflammation of the tissues present in lungs and thus can cause diseases such as lung cancer, silicosis and bronchitis.
-Silica finds its use in grinding and polishing stones and glasses, in manufacturing industries. It also finds its use as a gemstone.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




