
The bonds present in ${{\text{N}}_{2}}{{\text{O}}_{5}}$ are:
A. Ionic
B. Covalent and coordinate
C. Covalent
D. Ionic and covalent
Answer
528.4k+ views
Hint: The formation of bond take place by several methods such as ionic bond in which the complete transfer of electrons take place and a covalent bond is a bond which is formed by the sharing of the electrons and coordinate bond is the bond in which one element shares both the electrons.
Complete answer:
> Ionic bonds are formed between the metals and non-metals because the loss of an electron from an atom and gain of an electron by another atom takes place.
> Whereas in the covalent bond, the bond is formed between two metals and the equal sharing of electrons by both the elements take place.
> As we know that both nitrogen and oxygen are metals, so there are four covalent bonds present in nitrogen pentoxide.
> Moreover, we know that nitrogen can make three bonds as according to the electronic configuration of nitrogen (\[\text{1}{{\text{s}}^{2}}\text{ 2}{{\text{s}}^{2}}\text{ 2}{{\text{p}}^{3}}\]) there are three valence electrons in the outermost shell of the nitrogen.
> Also, nitrogen has a lone pair which does not participate in the bond formation and can be shared when needed and forms the coordinate bond.
> So, in nitrogen pentoxide, the structure includes four covalent bonds and one coordinate bond.
The structure of nitrogen pentoxide is:
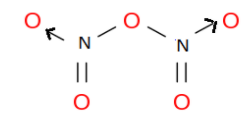
Here, the arrow represents the coordinate bond through which nitrogen shares a lone pair to the oxygen to fully fill its octet.
Therefore, option B is the correct answer.
Note: Generally non-metals form covalent bonds and metals form ionic bonds because they lose their electrons easily. Coordinate bond is also a type of covalent bond but here the electron comes from the same atom.
Complete answer:
> Ionic bonds are formed between the metals and non-metals because the loss of an electron from an atom and gain of an electron by another atom takes place.
> Whereas in the covalent bond, the bond is formed between two metals and the equal sharing of electrons by both the elements take place.
> As we know that both nitrogen and oxygen are metals, so there are four covalent bonds present in nitrogen pentoxide.
> Moreover, we know that nitrogen can make three bonds as according to the electronic configuration of nitrogen (\[\text{1}{{\text{s}}^{2}}\text{ 2}{{\text{s}}^{2}}\text{ 2}{{\text{p}}^{3}}\]) there are three valence electrons in the outermost shell of the nitrogen.
> Also, nitrogen has a lone pair which does not participate in the bond formation and can be shared when needed and forms the coordinate bond.
> So, in nitrogen pentoxide, the structure includes four covalent bonds and one coordinate bond.
The structure of nitrogen pentoxide is:

Here, the arrow represents the coordinate bond through which nitrogen shares a lone pair to the oxygen to fully fill its octet.
Therefore, option B is the correct answer.
Note: Generally non-metals form covalent bonds and metals form ionic bonds because they lose their electrons easily. Coordinate bond is also a type of covalent bond but here the electron comes from the same atom.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




