
Symbol of electric bulb is:
A.
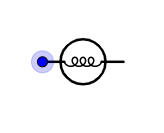
B.
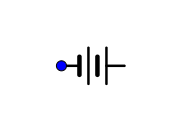
C.
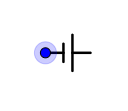
D.
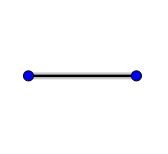




Answer
593.4k+ views
Hint: Here, we will proceed by defining the element inside a blub and the fact that it should be coiled. The element is tungsten. Then, we will mention the important properties of this element and due to those properties we will specify the use of this element.
Complete step by step answer:
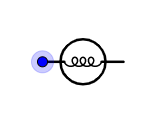 Is the symbol for an electric bulb.
Is the symbol for an electric bulb.
Hence the correct option is A.
Additional Information:
Tungsten (W) is also called wolfram. It is a chemical element which is an exceptionally strong refractory metal of Group 6 of the periodic table which is used in steels to increase the hardness and the strength and in lamp filaments.
Tungsten is known to be one of the toughest things found in nature. Its melting is almost impossible and they are super dense. Pure tungsten is a silver-white metal, which can be combustible and can ignite spontaneously when made into fine powder. Natural tungsten has five stable isotopes and an additional 21 unstable isotopes.
Tungsten is used in a variety of ways since it is very strong and durable. This is very corrosion resistant and has the highest melting point and maximum tensile strength of any element. However, its strength comes when it is transformed into compounds. Pure tungsten is too soft. Due to its high melting point, tungsten is used in the manufacture of the electric bulbs.
Note:
Tungsten has the highest melting point of all metals, and alloyed to reinforce them with other metals. Tungsten and its alloys are used in many applications of high temperatures, such as arc-welding electrodes and heating elements in high temperature furnaces. Tungsten carbide is incredibly hard and very important for the metalworking, mining and petroleum industries.
Complete step by step answer:

Hence the correct option is A.
Additional Information:
Tungsten (W) is also called wolfram. It is a chemical element which is an exceptionally strong refractory metal of Group 6 of the periodic table which is used in steels to increase the hardness and the strength and in lamp filaments.
Tungsten is known to be one of the toughest things found in nature. Its melting is almost impossible and they are super dense. Pure tungsten is a silver-white metal, which can be combustible and can ignite spontaneously when made into fine powder. Natural tungsten has five stable isotopes and an additional 21 unstable isotopes.
Tungsten is used in a variety of ways since it is very strong and durable. This is very corrosion resistant and has the highest melting point and maximum tensile strength of any element. However, its strength comes when it is transformed into compounds. Pure tungsten is too soft. Due to its high melting point, tungsten is used in the manufacture of the electric bulbs.
Note:
Tungsten has the highest melting point of all metals, and alloyed to reinforce them with other metals. Tungsten and its alloys are used in many applications of high temperatures, such as arc-welding electrodes and heating elements in high temperature furnaces. Tungsten carbide is incredibly hard and very important for the metalworking, mining and petroleum industries.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




