
Refer to the heating curve for ${H_2}O$ below.
Where is the temperature of ${H_2}O$ changing at$\dfrac{{{1^ \circ }{\text{C}}}}{{{\text{g}}{\text{.cal}}}}$?
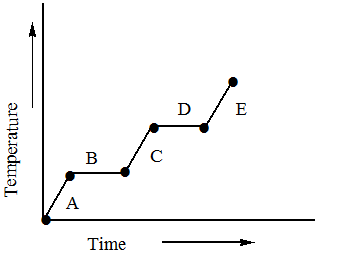
A.A
B.B
C.C
D.D
E.E

Answer
561.3k+ views
Hint: When water is heated up it goes through three phases which are solid, liquid and vapour. At the time of phase change there is no change in temperature as latent heat is being added to the molecules so that they can change their phase. Also the specific heat of water is $\dfrac{{{1^ \circ }{\text{C}}}}{{{\text{g}}{\text{.cal}}}}$ .
Complete step by step answer:
The point A represents that the water molecules which may be present in the form of ice are absorbing heat and as they are absorbing heat their temperature is being increased. At a certain point their temperature reaches $0^\circ C$ which is the melting point of the water.
At point B the latent heat of ice is being removed due to which no change in temperature is being observed and at the end of B the ice is converted to water.
At C, the water starts to absorb heat and its temperature starts to rise as it absorbs heat and at the end it reaches $100^\circ C$ .
At D, the water starts losing its latent heat of vaporization and starts to convert into the vapours. Since it is gaining latent heat there is no change in temperature observed in D.
At E the vapours are formed and heat is being absorbed by them and their temperature is being increased.
In question we are given the specific heat of water which is $\dfrac{{{1^ \circ }{\text{C}}}}{{{\text{g}}{\text{.cal}}}}$and this happens when the temperature is increasing in water state.
So, the correct answer is Option C.
Note: As we know that the water is having three phases which are solid (ice), liquid (water) and gas (vapour). The phase with most energy is vapour and the phase with least energy is ice. As we heat the ice it doesn’t convert into the liquid as soon as the temperature reaches $0^\circ C$ . At $0^\circ C$ the molecules take more energy just to break free the bonds and convert into water hence water has more energy than ice. Similarly, vapour has more energy than water at $100^\circ C$.
Complete step by step answer:
The point A represents that the water molecules which may be present in the form of ice are absorbing heat and as they are absorbing heat their temperature is being increased. At a certain point their temperature reaches $0^\circ C$ which is the melting point of the water.
At point B the latent heat of ice is being removed due to which no change in temperature is being observed and at the end of B the ice is converted to water.
At C, the water starts to absorb heat and its temperature starts to rise as it absorbs heat and at the end it reaches $100^\circ C$ .
At D, the water starts losing its latent heat of vaporization and starts to convert into the vapours. Since it is gaining latent heat there is no change in temperature observed in D.
At E the vapours are formed and heat is being absorbed by them and their temperature is being increased.
In question we are given the specific heat of water which is $\dfrac{{{1^ \circ }{\text{C}}}}{{{\text{g}}{\text{.cal}}}}$and this happens when the temperature is increasing in water state.
So, the correct answer is Option C.
Note: As we know that the water is having three phases which are solid (ice), liquid (water) and gas (vapour). The phase with most energy is vapour and the phase with least energy is ice. As we heat the ice it doesn’t convert into the liquid as soon as the temperature reaches $0^\circ C$ . At $0^\circ C$ the molecules take more energy just to break free the bonds and convert into water hence water has more energy than ice. Similarly, vapour has more energy than water at $100^\circ C$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




