
Pyridine is less basic than triethylamine because:
A. Pyridine has aromatic character
B. Nitrogen in pyridine is \[s{p^2}\] hybridised
C. Pyridine is a cyclic system
D. In pyridine, lone pair of nitrogen is delocalised
Answer
610.8k+ views
Hint: Pyridine is an aromatic compound. The structure of pyridine is similar to the structure of benzene in which a carbon atom is replaced by a nitrogen atom.
Complete step by step answer:
Pyridine is a heterocyclic organic compound with the chemical formula \[{C_5}{H_5}N\]. Pyridine is structurally related to benzene, with one methine group replaced by a nitrogen atom. Pyridine is actually colourless but some older and impure samples can appear yellow. In earlier days, pyridine was produced from coal tar.
The structure of pyridine is:
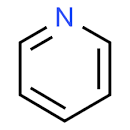
Pyridine is an aromatic cyclic structure in which the lone pair of electrons are not involved in resonance. Hence, the lone pair of electrons are not delocalised, but localised. The reason due to which pyridine is less basic than triethylamine is the presence of \[s{p^2}\] nitrogen in the structure of pyridine. The \[s{p^2}\] nitrogen is more electronegative than the \[s{p^3}\] nitrogen. Hence, the tendency of nitrogen to attract the electrons is more which reduces its electron donating nature to foreign species.
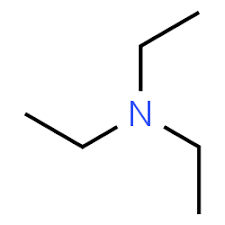
Triethylamine, on the other hand, has \[s{p^3}\] nitrogen as the central atom. Also, the ethyl groups increase the electron density on nitrogen, which in turn increases the electron donating ability of the central atom nitrogen. The structure of triethyl amine is:
Hence, pyridine is less basic than triethylamine due to the presence of \[s{p^2}\] nitrogen.
Therefore, the correct answer is (B).
Note: Remember that the \[s{p^2}\] carbons in the structure of pyridine also reduce the electronic donating ability of nitrogen. Triethyl amine has only \[s{p^3}\] carbons.
Complete step by step answer:
Pyridine is a heterocyclic organic compound with the chemical formula \[{C_5}{H_5}N\]. Pyridine is structurally related to benzene, with one methine group replaced by a nitrogen atom. Pyridine is actually colourless but some older and impure samples can appear yellow. In earlier days, pyridine was produced from coal tar.
The structure of pyridine is:

Pyridine is an aromatic cyclic structure in which the lone pair of electrons are not involved in resonance. Hence, the lone pair of electrons are not delocalised, but localised. The reason due to which pyridine is less basic than triethylamine is the presence of \[s{p^2}\] nitrogen in the structure of pyridine. The \[s{p^2}\] nitrogen is more electronegative than the \[s{p^3}\] nitrogen. Hence, the tendency of nitrogen to attract the electrons is more which reduces its electron donating nature to foreign species.
Triethylamine, on the other hand, has \[s{p^3}\] nitrogen as the central atom. Also, the ethyl groups increase the electron density on nitrogen, which in turn increases the electron donating ability of the central atom nitrogen. The structure of triethyl amine is:

Hence, pyridine is less basic than triethylamine due to the presence of \[s{p^2}\] nitrogen.
Therefore, the correct answer is (B).
Note: Remember that the \[s{p^2}\] carbons in the structure of pyridine also reduce the electronic donating ability of nitrogen. Triethyl amine has only \[s{p^3}\] carbons.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




