
\[{\text{PC}}{{\text{l}}_5}\] has the geometry:
A. Bipyramidal
B. Square planer
C. Planer
D. none
Answer
569.4k+ views
Hint:First we will determine the valence shell electronic configuration of the central atom. Then we will determine the steric number and then we will determine the hybridization and the geometry of the molecule.
Complete answer:
The chemical formula \[{\text{PC}}{{\text{l}}_5}\] represents the compound phosphorus pentachloride.
The central atom in \[{\text{PC}}{{\text{l}}_5}\] molecule is a phosphorus atom.
The atomic number of phosphorus is 15. The electronic configuration of phosphorus is \[\left[ {{\text{Ne}}} \right]{\text{ 3}}{{\text{s}}^2}{\text{3}}{{\text{p}}^3}\] . The phosphorus atom has five valence electrons. A phosphorus-chlorine bond is formed when one electron of phosphorus is shared with one electron of chlorine.
In phosphorus pentachloride molecules, the phosphorus atom forms five bonds with five chlorine atoms. Thus, all the five valence electrons of the central phosphorus atoms are consumed in formation of five phosphorus-chlorine bonds. Thus, the number of lone pairs of electrons on the phosphorus atom is zero. Thus, the central phosphorus atom has five bond pairs of electrons and zero lone pairs of electrons. The steric number of phosphorus is five.
The steric number of five is associated with \[s{p^3}d\] hybridization. One s, three d and on p atomic orbitals of central phosphorus atom undergo \[s{p^3}d\] hybridization to form five degenerate hybrid orbitals. These five degenerate hybrid orbitals overlap with atomic orbitals of five chlorine atoms to form five phosphorus-chlorine sigma covalent bonds.
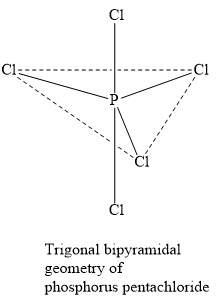
The steric number of five is associated with trigonal bipyramidal molecular geometry. Since the number of lone pairs of electrons is zero, the molecular geometry and the electron pair geometry are the same as trigonal bipyramidal.
Hence, the correct answer is option (A).
Note:The steric number gives the total number of bond pairs and lone pairs of electrons present around the central atom in a compound. For example, in the phosphorus pentachloride molecule, five bond pairs and zero lone pairs of electrons are present. Hence, the steric number is \[5 + 0 = 5\].
Complete answer:
The chemical formula \[{\text{PC}}{{\text{l}}_5}\] represents the compound phosphorus pentachloride.
The central atom in \[{\text{PC}}{{\text{l}}_5}\] molecule is a phosphorus atom.
The atomic number of phosphorus is 15. The electronic configuration of phosphorus is \[\left[ {{\text{Ne}}} \right]{\text{ 3}}{{\text{s}}^2}{\text{3}}{{\text{p}}^3}\] . The phosphorus atom has five valence electrons. A phosphorus-chlorine bond is formed when one electron of phosphorus is shared with one electron of chlorine.
In phosphorus pentachloride molecules, the phosphorus atom forms five bonds with five chlorine atoms. Thus, all the five valence electrons of the central phosphorus atoms are consumed in formation of five phosphorus-chlorine bonds. Thus, the number of lone pairs of electrons on the phosphorus atom is zero. Thus, the central phosphorus atom has five bond pairs of electrons and zero lone pairs of electrons. The steric number of phosphorus is five.
The steric number of five is associated with \[s{p^3}d\] hybridization. One s, three d and on p atomic orbitals of central phosphorus atom undergo \[s{p^3}d\] hybridization to form five degenerate hybrid orbitals. These five degenerate hybrid orbitals overlap with atomic orbitals of five chlorine atoms to form five phosphorus-chlorine sigma covalent bonds.
The steric number of five is associated with trigonal bipyramidal molecular geometry. Since the number of lone pairs of electrons is zero, the molecular geometry and the electron pair geometry are the same as trigonal bipyramidal.
Hence, the correct answer is option (A).

Note:The steric number gives the total number of bond pairs and lone pairs of electrons present around the central atom in a compound. For example, in the phosphorus pentachloride molecule, five bond pairs and zero lone pairs of electrons are present. Hence, the steric number is \[5 + 0 = 5\].
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




