
What is the oxidation state of Chromium (Cr) in ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$.
Answer
595.8k+ views
Hint: Oxidation state is the number of electrons that an atom needs to lose or gain to result in a chemical bond. To know the oxidation state of ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$, first we have to draw its structure so that we identify the type of oxygen atom.
Complete step-by-step answer:
The structure of ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$is,
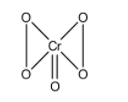
In the compounds, four oxygen atoms are part of peroxide linkage and we know that in peroxides the oxidation state of oxygen is -1. And in all other compounds, the oxidation state of oxygen atom is -2.
If we know the type of compound, we will easily know the oxidation number of compounds. For neutral compounds, such as ${{\rm{H}}_{\rm{2}}}{\rm{O}}$, ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$, etc. the addition of oxidation state of all atoms of the compound is equal to zero.
So, in the compound, ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$, four peroxide oxygen atoms has oxidation state of -1 each and the remaining oxygen has oxidation state of -2. Using these values, now we calculate the oxidation state of chromium.
We take x as the oxidation state of chromium in ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$.
$\begin{array}{c}x + \left( { - 1 \times 4} \right) + \left( { - 2} \right) = 0\\x - 4 - 2 = 0\\x = 6\end{array}$
Hence, the oxidation state of chromium in ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$ is 6.
Additional Information:
1) If a compound exists in elemental form (only one type of atoms present), the oxidation number of the element is always zero.
2) For ions, the charge indicates the oxidation number. For example, the oxidation number of chloride ions is -1.
Note: Students have to take care of taking the oxidation number of oxygen in the calculation of an oxidation number of an element in a compound. In peroxides, oxygen possesses oxidation state of -1. In ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$ four oxygen atoms has -1 oxidation state and the remaining one oxygen atom possesses oxidation state of -2.
Complete step-by-step answer:
The structure of ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$is,

In the compounds, four oxygen atoms are part of peroxide linkage and we know that in peroxides the oxidation state of oxygen is -1. And in all other compounds, the oxidation state of oxygen atom is -2.
If we know the type of compound, we will easily know the oxidation number of compounds. For neutral compounds, such as ${{\rm{H}}_{\rm{2}}}{\rm{O}}$, ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$, etc. the addition of oxidation state of all atoms of the compound is equal to zero.
So, in the compound, ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$, four peroxide oxygen atoms has oxidation state of -1 each and the remaining oxygen has oxidation state of -2. Using these values, now we calculate the oxidation state of chromium.
We take x as the oxidation state of chromium in ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$.
$\begin{array}{c}x + \left( { - 1 \times 4} \right) + \left( { - 2} \right) = 0\\x - 4 - 2 = 0\\x = 6\end{array}$
Hence, the oxidation state of chromium in ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$ is 6.
Additional Information:
1) If a compound exists in elemental form (only one type of atoms present), the oxidation number of the element is always zero.
2) For ions, the charge indicates the oxidation number. For example, the oxidation number of chloride ions is -1.
Note: Students have to take care of taking the oxidation number of oxygen in the calculation of an oxidation number of an element in a compound. In peroxides, oxygen possesses oxidation state of -1. In ${\rm{Cr}}{{\rm{O}}_{\rm{5}}}$ four oxygen atoms has -1 oxidation state and the remaining one oxygen atom possesses oxidation state of -2.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




