
List four characteristics of a mixture.
Answer
575.7k+ views
Hint:When two or substance mix in a ratio, a mixture forms. The substances of the mixture are known as its components. The components of a mixture can be mixed completely to give a homogeneous mixture. The components of a mixture can be mixed unevenly to give a heterogeneous mixture.
Complete answer
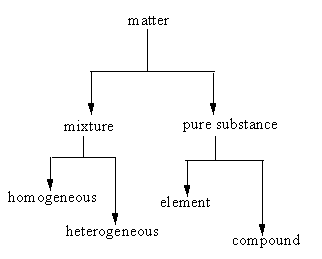
Anything which occupies space and has mass is known as matter. The matter is divided into two types: Mixture and pure substance.
Mixture: a mixture contains two or more substances in a ratio.
Mixture further can be of two types:
i) Homogenous: In a homogeneous mixture, components mix very well into each other, so the mixture looks even.
ii) Heterogeneous: In a heterogeneous mixture, components do not mix very well into each other, so the mixture looks uneven.
The following chart represents the types in which the matter is divided.
The properties of the mixture are as follows:
>The mixture has various components in a ratio but not in a fixed ratio such as sugar solution. In one glass of water, we can dissolve one spoon of sugar or two spoons of sugar.
>The melting and boiling point of the mixture is not fixed. As it contains many components which can have different melting and boiling temperatures.
>By using a simple physical method such as handpicking, distillation, crystallization, and filtration, the components of the mixture can be separated.
>Energy is not necessary to form a mixture and energy also does not evolve by the formation of a mixture.
Note: Pure substance has a fixed composition. A pure substance is further divided into three parts: Element: elements are made up of only one type of atoms or molecules. Compound: compounds are made up of different types of atoms or molecules. The composition of the mixture is variable whereas the composition of a pure substance is fixed. The ore which contains different metals and various impurities is an example of a mixture. Carbohydrates are an example of pure substance. Pure substances cannot be separated by simple physical separation methods.
Complete answer
Anything which occupies space and has mass is known as matter. The matter is divided into two types: Mixture and pure substance.
Mixture: a mixture contains two or more substances in a ratio.
Mixture further can be of two types:
i) Homogenous: In a homogeneous mixture, components mix very well into each other, so the mixture looks even.
ii) Heterogeneous: In a heterogeneous mixture, components do not mix very well into each other, so the mixture looks uneven.
The following chart represents the types in which the matter is divided.

The properties of the mixture are as follows:
>The mixture has various components in a ratio but not in a fixed ratio such as sugar solution. In one glass of water, we can dissolve one spoon of sugar or two spoons of sugar.
>The melting and boiling point of the mixture is not fixed. As it contains many components which can have different melting and boiling temperatures.
>By using a simple physical method such as handpicking, distillation, crystallization, and filtration, the components of the mixture can be separated.
>Energy is not necessary to form a mixture and energy also does not evolve by the formation of a mixture.
Note: Pure substance has a fixed composition. A pure substance is further divided into three parts: Element: elements are made up of only one type of atoms or molecules. Compound: compounds are made up of different types of atoms or molecules. The composition of the mixture is variable whereas the composition of a pure substance is fixed. The ore which contains different metals and various impurities is an example of a mixture. Carbohydrates are an example of pure substance. Pure substances cannot be separated by simple physical separation methods.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Trending doubts
What is BLO What is the full form of BLO class 8 social science CBSE

Citizens of India can vote at the age of A 18 years class 8 social science CBSE

The number of corners in a cube are A 4 B 6 C 8 D class 8 maths CBSE

What are the methods of reducing friction. Explain

Advantages and disadvantages of science

Which part of nephron allows the selective reabsorption class 8 biology CBSE




