
What is isomerism? Name the isomers of butane.
Answer
595.8k+ views
Hint: When two compounds have similar molecular formulas but different structures, they are called isomers of each other. 4 Carbon atoms are present in butane which can be arranged in two different ways.
Complete step by step answer: Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures. When the atoms and the functional groups are joined together in different ways but the molecular formula remains the same, the type of isomerism is called Structural isomerism. Butane is an alkane with molecular formula \[{{\text{C}}_4}{{\text{H}}_{10}}\]. Following types of structures are possible with 4 Carbon atoms.
- n-butane or straight chain butane

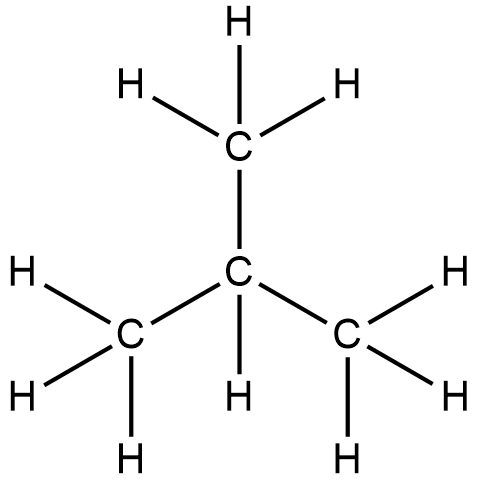
- 2-methyl propane or isobutane
Additional information: There are a few types of structural isomerism:
- Chain Isomerism
When the carbon molecules in the chain of an organic molecule are relocated but the molecular formulas remain the same, Chain isomerism is confirmed. This alters the chain of the organic compound. The number of possible structural isomers increases greatly with the number of available atoms.
- Position isomerism
When compounds having the same molecular formula, same structural formula but the position of functional groups between two identical organic compounds is different, the compounds are said to show positional isomerism.
- Functional isomerism
Organic compounds which have the same molecular formula but the functional group present is different are called functional isomers of each other and the phenomenon is called functional isomerism.
Note: Isomers may or may not have the same chemical properties. For example - stereoisomerism like geometrical isomerism, the chemical properties of two isomers are different.
Complete step by step answer: Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures. When the atoms and the functional groups are joined together in different ways but the molecular formula remains the same, the type of isomerism is called Structural isomerism. Butane is an alkane with molecular formula \[{{\text{C}}_4}{{\text{H}}_{10}}\]. Following types of structures are possible with 4 Carbon atoms.
- n-butane or straight chain butane

- 2-methyl propane or isobutane
In which there are 3 carbon atoms in the parent chain.

Additional information: There are a few types of structural isomerism:
- Chain Isomerism
When the carbon molecules in the chain of an organic molecule are relocated but the molecular formulas remain the same, Chain isomerism is confirmed. This alters the chain of the organic compound. The number of possible structural isomers increases greatly with the number of available atoms.
- Position isomerism
When compounds having the same molecular formula, same structural formula but the position of functional groups between two identical organic compounds is different, the compounds are said to show positional isomerism.
- Functional isomerism
Organic compounds which have the same molecular formula but the functional group present is different are called functional isomers of each other and the phenomenon is called functional isomerism.
Note: Isomers may or may not have the same chemical properties. For example - stereoisomerism like geometrical isomerism, the chemical properties of two isomers are different.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




