
In the following reactions, what will be A, B and C respectively?
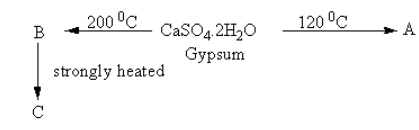
A. Plaster of Paris, dead burnt plaster, calcium sulphide
B. dead burnt plaster, plaster of Paris, lime
C. Plaster of Paris, dead burnt plaster, lime
D. Anhydrous calcium sulphate, Plaster of Paris, calcium sulfite
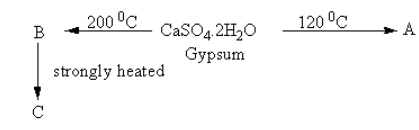
Answer
569.7k+ views
Hint: When calcium sulphate dihydrate is heated at low temperature, some amount of water escapes from the calcium sulphate. On increasing temperature, complete water escapes from calcium sulphate dihydrate. When anhydrous calcium sulphate is strongly heated it converts into an oxide of calcium.
Complete Step by step answer:When calcium sulphate dihydrate is heated at ${\text{120}}{\,^{\text{o}}}{\text{C}}$ , some amount of water escapes from the calcium sulphate.
The reaction is as follows:
\[{\text{CaS}}{{\text{O}}_{\text{4}}}{\text{.2}}{{\text{H}}_{\text{2}}}{\text{O}}\,\mathop \to \limits^{{\text{120}}{\,^{\text{o}}}{\text{C}}} \,\mathop {{\text{CaS}}{{\text{O}}_{\text{4}}}\,.\dfrac{1}{2}{{\text{H}}_{\text{2}}}{\text{O}}}\limits_{\text{A}} + \dfrac{3}{2}{{\text{H}}_{\text{2}}}{\text{O}}\]
The calcium sulphate having half water molecule \[\left( {{\text{CaS}}{{\text{O}}_{\text{4}}}\,.\dfrac{1}{2}{{\text{H}}_{\text{2}}}{\text{O}}} \right)\]is known as Plaster of Paris.
When calcium sulphate dihydrate is heated at ${\text{200}}{\,^{\text{o}}}{\text{C}}$ , complete water escapes from the calcium sulphate.
The reaction is as follows:
\[{\text{CaS}}{{\text{O}}_{\text{4}}}{\text{.2}}{{\text{H}}_{\text{2}}}{\text{O}}\,\mathop \to \limits^{{\text{200}}{\,^{\text{o}}}{\text{C}}} \mathop {\,{\text{CaS}}{{\text{O}}_{\text{4}}}\,}\limits_{\text{B}} + 2\,{{\text{H}}_{\text{2}}}{\text{O}}\]
The anhydrous calcium sulphate\[\left( {{\text{CaS}}{{\text{O}}_{\text{4}}}} \right)\] is known as dead burnt plaster.
When anhydrous calcium sulphate is heated strongly, it dissociates into calcium oxide and sulphur trioxide.
The reaction is as the temperature of the heating is important. At low temperature, some amount of water remains in compound calcium sulphate which is known as Plaster of Paris whereas at high temperature all the water removes which forms the same compound but have different properties.follows:
\[{\text{2}}\,{\text{CaS}}{{\text{O}}_{\text{4}}}\,\mathop \to \limits^{{\text{Strongly}}\,{\text{heated}}} \mathop {\,2\,{\text{CaO}}\,}\limits_{\text{C}} + 2\,{\text{S}}{{\text{O}}_3}\]
The calcium oxide\[\left( {{\text{CaO}}} \right)\] is known as lime.
So, product A is Plaster of Paris, product B is dead burnt plaster and product C is lime.
The calcium sulphide does not form during any reaction, so option (A) is incorrect.
The products are not arranged in the correct order so, option (B) is incorrect.
The products are arranged in the correct order so, option (C) is correct.
The calcium sulphite does not form during any reaction, so option (D) is incorrect.
Therefore, option (C) Plaster of Paris, dead burnt plaster, lime, is correct.
Note: The temperature of the heating is important. At low temperature, some amount of water remains in compound calcium sulphate which is known as Plaster of Paris whereas at high temperature all the water removes which forms the same compound but have different properties.
Complete Step by step answer:When calcium sulphate dihydrate is heated at ${\text{120}}{\,^{\text{o}}}{\text{C}}$ , some amount of water escapes from the calcium sulphate.
The reaction is as follows:
\[{\text{CaS}}{{\text{O}}_{\text{4}}}{\text{.2}}{{\text{H}}_{\text{2}}}{\text{O}}\,\mathop \to \limits^{{\text{120}}{\,^{\text{o}}}{\text{C}}} \,\mathop {{\text{CaS}}{{\text{O}}_{\text{4}}}\,.\dfrac{1}{2}{{\text{H}}_{\text{2}}}{\text{O}}}\limits_{\text{A}} + \dfrac{3}{2}{{\text{H}}_{\text{2}}}{\text{O}}\]
The calcium sulphate having half water molecule \[\left( {{\text{CaS}}{{\text{O}}_{\text{4}}}\,.\dfrac{1}{2}{{\text{H}}_{\text{2}}}{\text{O}}} \right)\]is known as Plaster of Paris.
When calcium sulphate dihydrate is heated at ${\text{200}}{\,^{\text{o}}}{\text{C}}$ , complete water escapes from the calcium sulphate.
The reaction is as follows:
\[{\text{CaS}}{{\text{O}}_{\text{4}}}{\text{.2}}{{\text{H}}_{\text{2}}}{\text{O}}\,\mathop \to \limits^{{\text{200}}{\,^{\text{o}}}{\text{C}}} \mathop {\,{\text{CaS}}{{\text{O}}_{\text{4}}}\,}\limits_{\text{B}} + 2\,{{\text{H}}_{\text{2}}}{\text{O}}\]
The anhydrous calcium sulphate\[\left( {{\text{CaS}}{{\text{O}}_{\text{4}}}} \right)\] is known as dead burnt plaster.
When anhydrous calcium sulphate is heated strongly, it dissociates into calcium oxide and sulphur trioxide.
The reaction is as the temperature of the heating is important. At low temperature, some amount of water remains in compound calcium sulphate which is known as Plaster of Paris whereas at high temperature all the water removes which forms the same compound but have different properties.follows:
\[{\text{2}}\,{\text{CaS}}{{\text{O}}_{\text{4}}}\,\mathop \to \limits^{{\text{Strongly}}\,{\text{heated}}} \mathop {\,2\,{\text{CaO}}\,}\limits_{\text{C}} + 2\,{\text{S}}{{\text{O}}_3}\]
The calcium oxide\[\left( {{\text{CaO}}} \right)\] is known as lime.
So, product A is Plaster of Paris, product B is dead burnt plaster and product C is lime.
The calcium sulphide does not form during any reaction, so option (A) is incorrect.
The products are not arranged in the correct order so, option (B) is incorrect.
The products are arranged in the correct order so, option (C) is correct.
The calcium sulphite does not form during any reaction, so option (D) is incorrect.
Therefore, option (C) Plaster of Paris, dead burnt plaster, lime, is correct.
Note: The temperature of the heating is important. At low temperature, some amount of water remains in compound calcium sulphate which is known as Plaster of Paris whereas at high temperature all the water removes which forms the same compound but have different properties.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




