
(i) Write the structural formula of acetic acid.
(ii) Write the electron dot structure of ethane.
(iii) Explain an odd atom by an example.
(iv) Select unsaturated hydrocarbon in ${{C}_{2}}{{H}_{6}}$,${{C}_{3}}{{H}_{4}}$, ${{C}_{3}}{{H}_{8}}$and ${{C}_{2}}{{H}_{4}}$.
(v) Write a difference between soap and detergents.
Answer
601.2k+ views
Hint: (i) It contains two carbon atoms. It has a carboxyl group.
(ii) First draw the structural formula. Then add the dots to each bond.
(iii) The characters of this type of atom are as its name suggests. These atoms have an odd number of electrons when present in a molecule.
(iv) Unsaturated hydrocarbon contains double and triple bonds. They have less number of hydrogens than a saturated molecule.
(v) Soaps and detergents are chemically different but functionally similar. Soaps are milder than detergents.
Complete answer:
(i) Acetic acid is the common name of ethanoic acid. Ethanoic acid has two carbon atoms. We come to know this fact because of the suffix “ethan” which relates to ethane. Ethane is an alkane with two carbon atoms and six hydrogen atoms. Ethanoic acid also should contain a carboxylic acid functional group. The structural formula is therefore as below:
\[C{{H}_{3}}COOH\]
(ii) Ethane as mentioned above contains two carbons and is an alkane by nature. The general formula of an alkane is ${{C}_{n}}{{H}_{2n+2}}$. So the number of hydrogens is six. As an alkane contains all single bonds, below is the structure of ethane and adjacent to it is its electron dot structure:
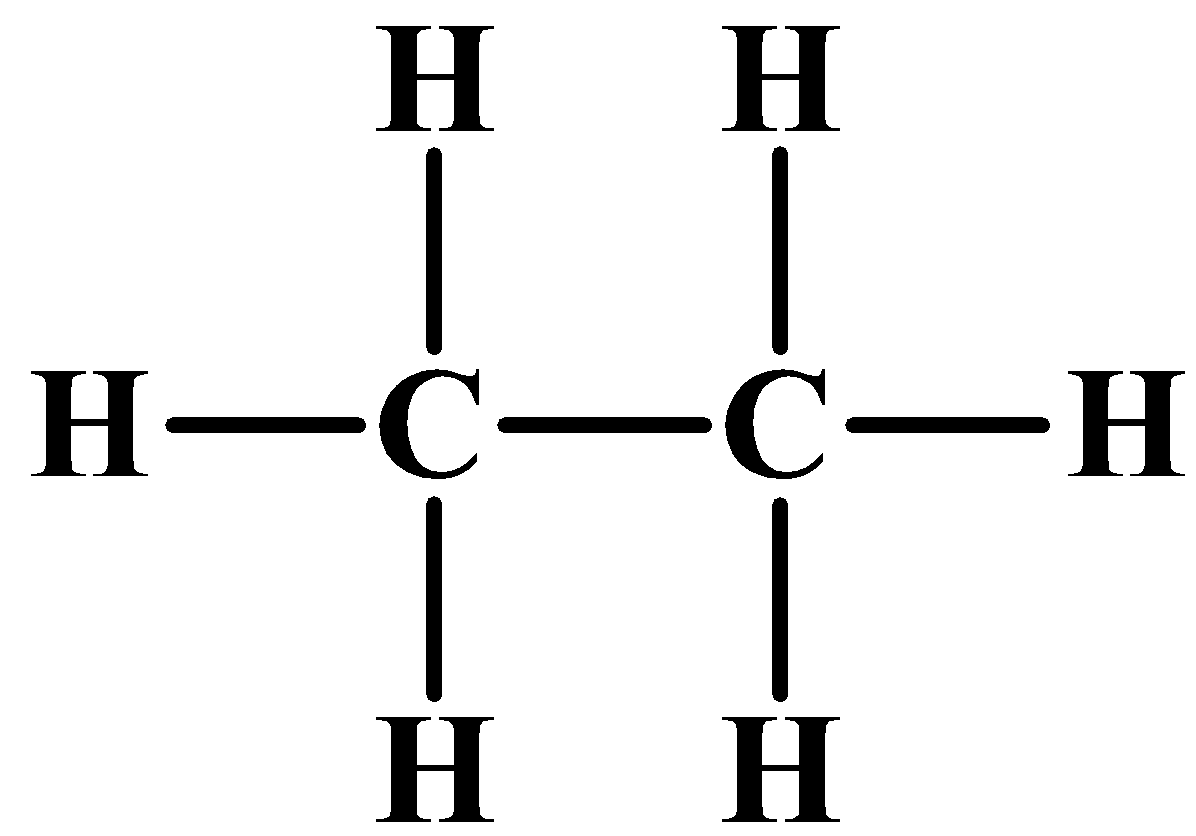
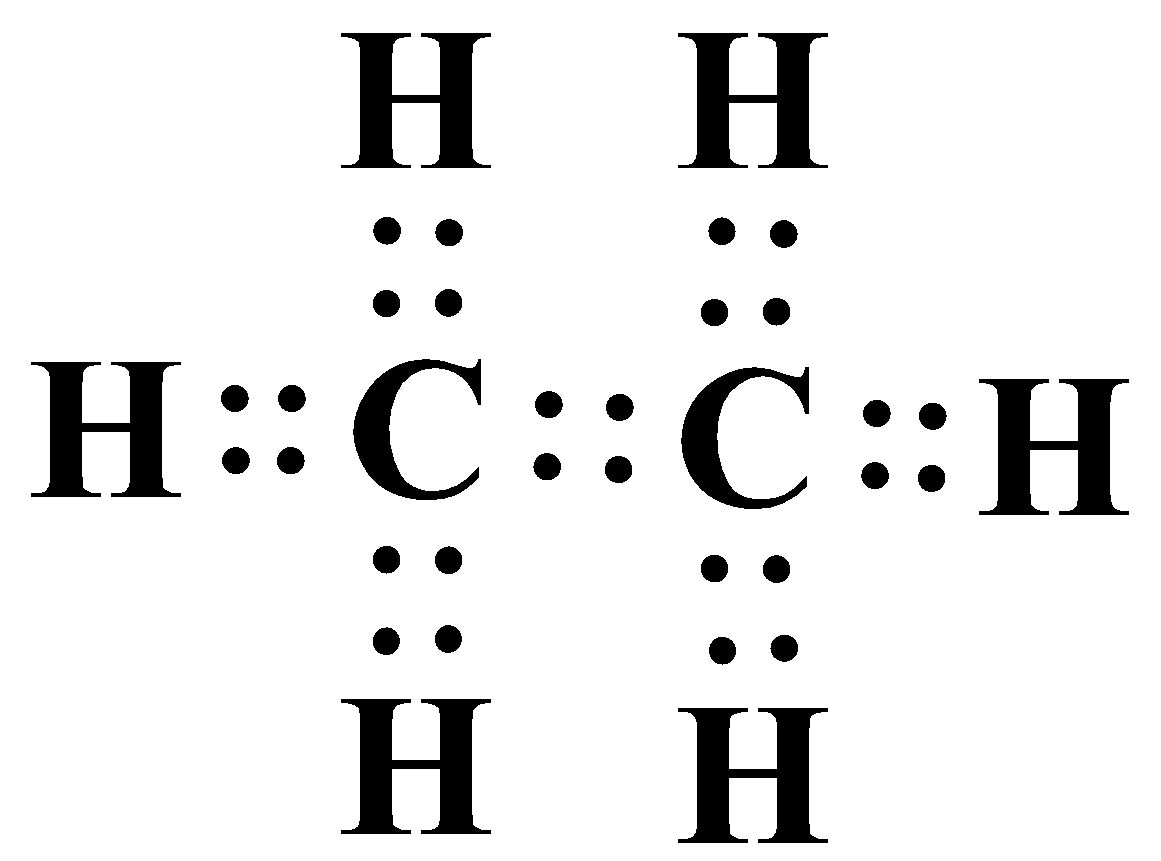
(iii) The odd electron atom is related to the exception of certain atoms to the octet rule which states that the valence shell of atoms (except some) should be filled with eight electrons so as to satisfy their molecular states. But molecules like nitrogen dioxide do not satisfy their octet but are still stable. Its molecular structure is as below:
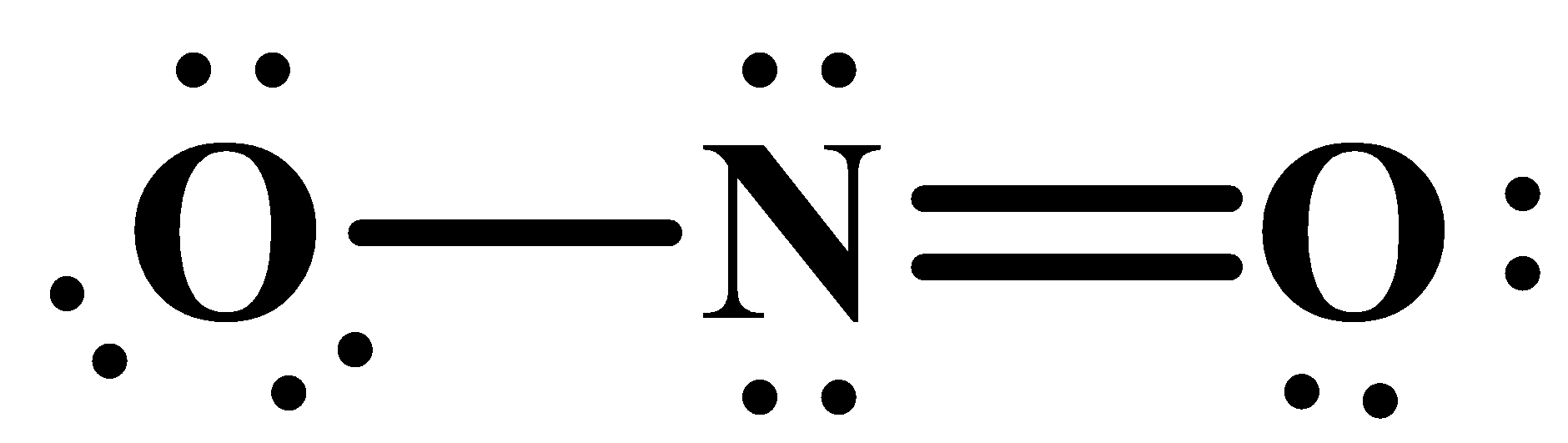
As you can see here, the oxygen atom has an excess of electrons and therefore carries a negative charge. The free radicals are also an exception to the octet rule.
(iv) We have been given the following hydrocarbons:
${{C}_{2}}{{H}_{6}}$,${{C}_{3}}{{H}_{4}}$, ${{C}_{3}}{{H}_{8}}$and ${{C}_{2}}{{H}_{4}}$
And we have to find the unsaturated ones. If the compound is an alkane, then it is saturated and all the rest are unsaturated. The general formula of alkane is ${{C}_{n}}{{H}_{2n+2}}$. Only ${{C}_{2}}{{H}_{6}}$ and ${{C}_{3}}{{H}_{8}}$ satisfy the conditions and therefore they are alkane and saturated. The other compounds, that is, ${{C}_{3}}{{H}_{4}}$ and ${{C}_{2}}{{H}_{4}}$ are unsaturated.
(v)
Note:
The dot structure of any molecule should contain all the electrons that are owned or shared by the atoms.
The octet rule does not apply to molecules after the second period. The valence shells do not always have eight electrons. The electronic configurations are more appropriate when they are written in accordance with the shells, subshells and orbitals.
(ii) First draw the structural formula. Then add the dots to each bond.
(iii) The characters of this type of atom are as its name suggests. These atoms have an odd number of electrons when present in a molecule.
(iv) Unsaturated hydrocarbon contains double and triple bonds. They have less number of hydrogens than a saturated molecule.
(v) Soaps and detergents are chemically different but functionally similar. Soaps are milder than detergents.
Complete answer:
(i) Acetic acid is the common name of ethanoic acid. Ethanoic acid has two carbon atoms. We come to know this fact because of the suffix “ethan” which relates to ethane. Ethane is an alkane with two carbon atoms and six hydrogen atoms. Ethanoic acid also should contain a carboxylic acid functional group. The structural formula is therefore as below:
\[C{{H}_{3}}COOH\]
(ii) Ethane as mentioned above contains two carbons and is an alkane by nature. The general formula of an alkane is ${{C}_{n}}{{H}_{2n+2}}$. So the number of hydrogens is six. As an alkane contains all single bonds, below is the structure of ethane and adjacent to it is its electron dot structure:


(iii) The odd electron atom is related to the exception of certain atoms to the octet rule which states that the valence shell of atoms (except some) should be filled with eight electrons so as to satisfy their molecular states. But molecules like nitrogen dioxide do not satisfy their octet but are still stable. Its molecular structure is as below:
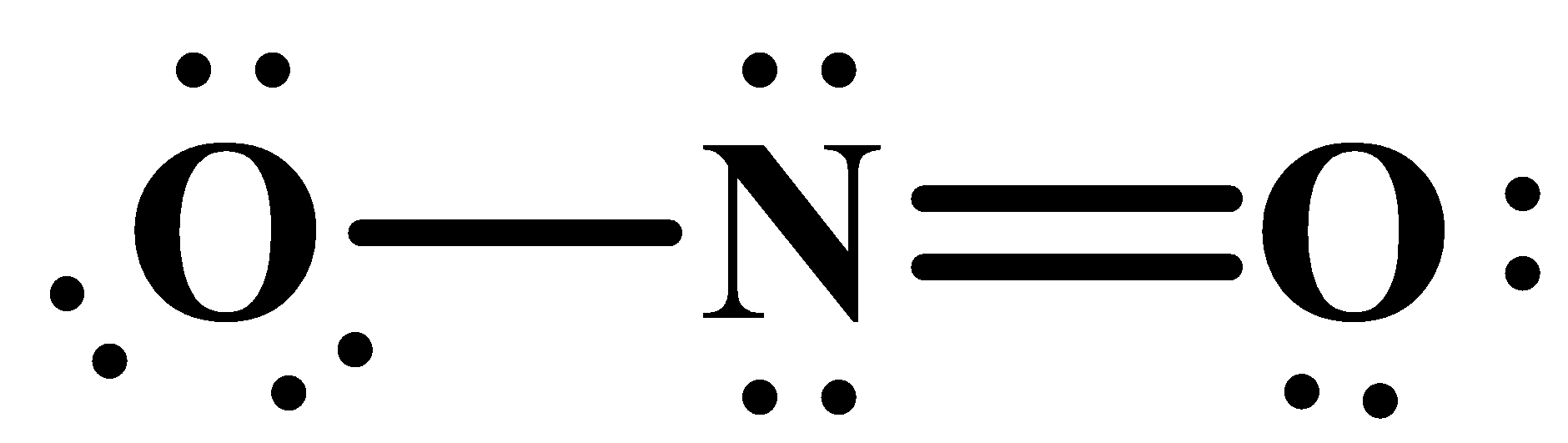
As you can see here, the oxygen atom has an excess of electrons and therefore carries a negative charge. The free radicals are also an exception to the octet rule.
(iv) We have been given the following hydrocarbons:
${{C}_{2}}{{H}_{6}}$,${{C}_{3}}{{H}_{4}}$, ${{C}_{3}}{{H}_{8}}$and ${{C}_{2}}{{H}_{4}}$
And we have to find the unsaturated ones. If the compound is an alkane, then it is saturated and all the rest are unsaturated. The general formula of alkane is ${{C}_{n}}{{H}_{2n+2}}$. Only ${{C}_{2}}{{H}_{6}}$ and ${{C}_{3}}{{H}_{8}}$ satisfy the conditions and therefore they are alkane and saturated. The other compounds, that is, ${{C}_{3}}{{H}_{4}}$ and ${{C}_{2}}{{H}_{4}}$ are unsaturated.
(v)
| SOAPS | DETERGENTS |
1. These are composed of metal salts of long-chain fatty acids.2. They are less soluble in hard water.3. These are weaker cleansing agents and are therefore safe to be applied on our skins.4. The structure of a molecule that constitutes soap: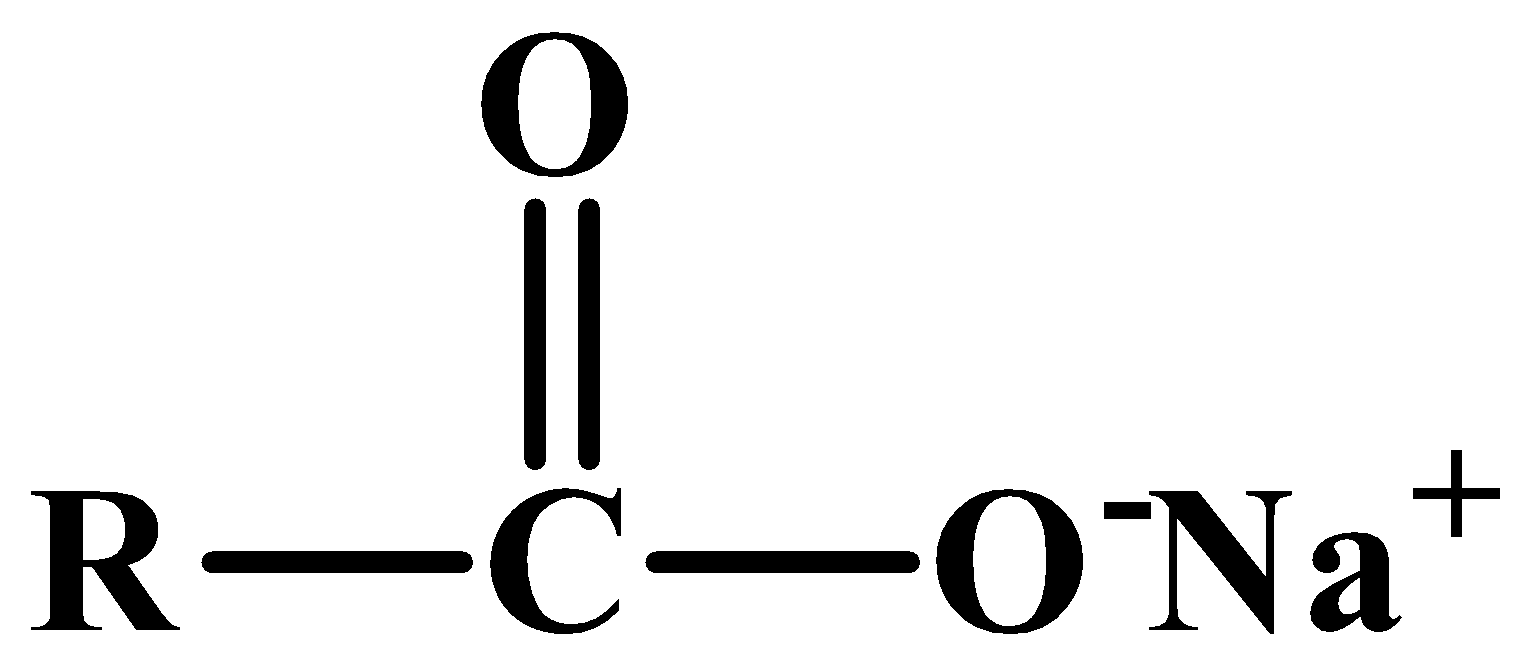
| 1. These are composed of metal salts of alkyl benzene sulfonates.2. They are more soluble in hard water.3. These are stronger cleansing agents and therefore should not be applied to human skin.4. The structure of a molecule that constitutes detergent: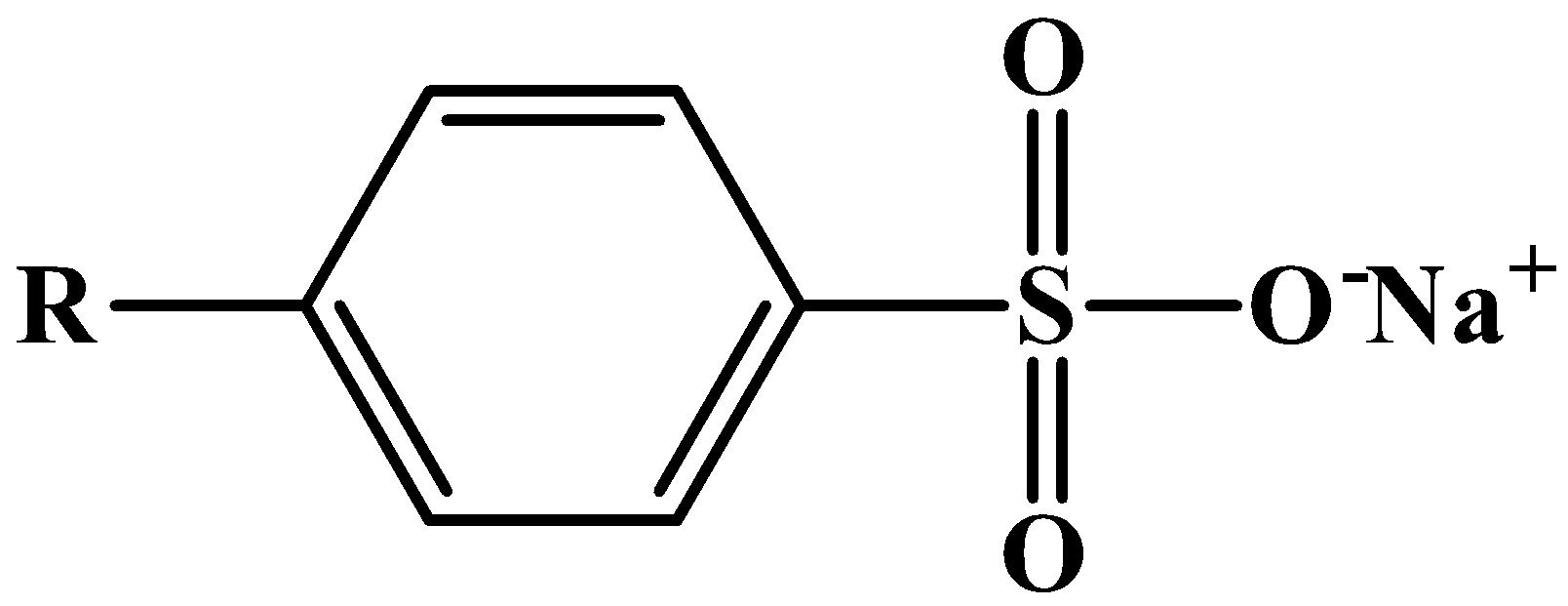
|
Note:
The dot structure of any molecule should contain all the electrons that are owned or shared by the atoms.
The octet rule does not apply to molecules after the second period. The valence shells do not always have eight electrons. The electronic configurations are more appropriate when they are written in accordance with the shells, subshells and orbitals.
Recently Updated Pages
Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Computer Science: Engaging Questions & Answers for Success

Class 10 Question and Answer - Your Ultimate Solutions Guide

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Trending doubts
A boat goes 24 km upstream and 28 km downstream in class 10 maths CBSE

State and explain Ohms law class 10 physics CBSE

Distinguish between soap and detergent class 10 chemistry CBSE

a Why did Mendel choose pea plants for his experiments class 10 biology CBSE

What is a "free hit" awarded for in limited-overs cricket?

Draw the diagram of the sectional view of the human class 10 biology CBSE




