
Give resonating structures of Anisole.
Answer
602.4k+ views
Hint: We must know that the resonance structures are series of Lewis structures which represent electronic bonding on structure. Therefore, resonating is a process that’s stabilizing the chemical structure by shifting electrons.
Complete step by step solution:
We must remember that a molecule can have resonating structures only when it carries a lone pair or double bond on the atom that is next to the double bond in a molecule. And also the concept of the conjugation of “double bond - single bond – double bond” in benzene ring or “double bond – single bond – lone pair” conjugation in the benzene ring.
Anisole, also known as methoxy-benzene, has a chemical formula as \[C{H_3}O{C_6}{H_5}\].
If we can draw more than one correct Lewis structure that satisfies octet rule for a molecule, then the structure of that molecule is known as resonating structures.
Resonating structures have two or more possible structures that can be represented by dotted lines or with an arrow between them.
Changing the position of the double bond may give you the final product of resonance. Moving lone pairs and bonds will give you intermediate structures of resonance.
Following are resonating structures of Anisole:
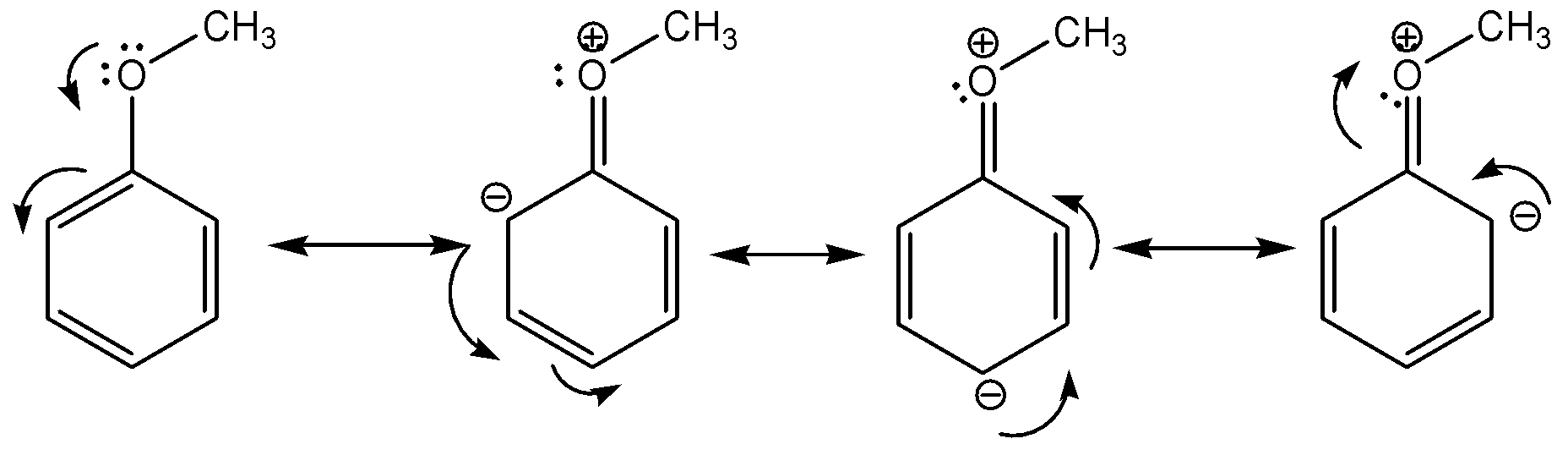
Additional information:we must remember that the resonating structures of a molecule represent the delocalization of electrons within the molecule itself and so the structures are also known as resonance hybrids. Methoxy group in anisole is an electron-donating group and it is directly attached to the benzene ring. Due to the resonance donation of electrons by the methoxy group, Anisole is more reactive in electrophilic aromatic substitution.
Note: We need to remember these points while drawing resonating structures:
Never move atoms in the structure.
Move only electrons in lone pair and double bond
Keep the framework of bond in molecules intact
While drawing a resonating structure we keep the overall charge of the molecule to be the same.
Complete step by step solution:
We must remember that a molecule can have resonating structures only when it carries a lone pair or double bond on the atom that is next to the double bond in a molecule. And also the concept of the conjugation of “double bond - single bond – double bond” in benzene ring or “double bond – single bond – lone pair” conjugation in the benzene ring.
Anisole, also known as methoxy-benzene, has a chemical formula as \[C{H_3}O{C_6}{H_5}\].
If we can draw more than one correct Lewis structure that satisfies octet rule for a molecule, then the structure of that molecule is known as resonating structures.
Resonating structures have two or more possible structures that can be represented by dotted lines or with an arrow between them.
Changing the position of the double bond may give you the final product of resonance. Moving lone pairs and bonds will give you intermediate structures of resonance.
Following are resonating structures of Anisole:

Additional information:we must remember that the resonating structures of a molecule represent the delocalization of electrons within the molecule itself and so the structures are also known as resonance hybrids. Methoxy group in anisole is an electron-donating group and it is directly attached to the benzene ring. Due to the resonance donation of electrons by the methoxy group, Anisole is more reactive in electrophilic aromatic substitution.
Note: We need to remember these points while drawing resonating structures:
Never move atoms in the structure.
Move only electrons in lone pair and double bond
Keep the framework of bond in molecules intact
While drawing a resonating structure we keep the overall charge of the molecule to be the same.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




