
For the reaction,
\[SO_2\left( g \right){\text{ }} + {\text{ }}NO_2\left( g \right)\;\; \rightleftharpoons \;SO_3\left( g \right){\text{ }} + {\text{ }}NO\left( g \right)\;\] \[Kc{\text{ }} = {\text{ }}16\].
The reaction is carried out in a closed container at a fixed temperature. Find the concentration of \[NO\] and \[N{O_2}\] at equilibrium if \[1{\text{ }}mol\]of each reacts in a container of \[1{\text{ }}liter\] capacity.
Answer
594.9k+ views
Hint: We have to find the concentration of \[NO\]and at equilibrium. So we can use the equilibrium constant formula to solve the problem,
\[Kc = \dfrac{{{{\left[ C \right]}^c}{{\left[ D \right]}^d}}}{{{{\left[ A \right]}^a}{{\left[ B \right]}^b}}}\]
Complete step by step solution:
We can define the equilibrium constant \[(Kc)\]is the ratio of the equilibrium concentrations of products upon the equilibrium concentrations of reactants and that each reactant and product raised to the power of their respective coefficients.
Thus,the equilibrium constant,\[Kc\] , for the reaction \[{\text{aA + bB }} \rightleftharpoons {\text{ cC + dD}}\] is given as,
\[Kc{\text{ }} = \]\[\dfrac{{{{\left[ C \right]}^c}{{\left[ D \right]}^d}}}{{{{\left[ A \right]}^a}{{\left[ B \right]}^b}}}\]
where A and B are reactants and C &D are products. a,b,c and d are their coefficients respectively.
Given,
Volume Of container = \[1{\text{ }}Liter\]
Equilibriumconstant = \[Kc{\text{ }} = {\text{ }}16\]
The equilibrium reaction is \[{\text{SO_2}}\left( {\text{g}} \right){\text{ + NO_2}}\left( {\text{g}} \right){\text{}} \rightleftharpoons {\text{SO_3}}\left( {\text{g}} \right){\text{ + NO}}\left( {\text{g}} \right){\text{}}\]
of each of the four gases given is \[1{\text{ }}M\]each,then
Now, by applying the formula for the expression for the equilibrium constant,
\[Kc = \]\[\dfrac{{{{\left[ {SO_3} \right]}^{}}{{\left[ {NO} \right]}^{}}}}{{{{\left[ {SO_2} \right]}^{}}{{\left[ {NO_2} \right]}^{}}}}\]
Putting the values of equilibrium concentrations of reactants and products ,we get
\[Kc = \] \[\dfrac{{\left[ {1 + x} \right]\left[ {1 + x} \right]}}{{\left[ {1 - x} \right]\left[ {1 - x} \right]}}\] As \[Kc\] is 16 , then by solving the above we get,
∴ \[16 = \dfrac{{{{\left( {1 + x} \right)}^2}}}{{{{\left( {1 - x} \right)}^2}}}\]
∴ \[4 = \dfrac{{\left( {1 + x} \right)}}{{\left( {1 - x} \right)}}\] after simplifying above
∴ \[x{\text{ }} = {\text{ }}0.6\] By substituting the value of x ,
\[\left[ {S{O_3}} \right]{\text{ }} = {\text{ }}\left[ {NO} \right]{\text{ }} = {\text{ }}\left( {1 + {\text{ }}x} \right){\text{ }} = {\text{ }}1 + 0.6{\text{ }} = {\text{ }}1.6{\text{ }}mol/L\]
\[\left[ {S{O_2}} \right]{\text{ }} = {\text{ }}\left[ {N{O_2}} \right]{\text{ }} = {\text{ }}\left( {1 - {\text{ }}x} \right){\text{ }} = {\text{ }}1{\text{ }} - {\text{ }}0.6{\text{ }} = {\text{ }}0.4{\text{ }}mol/L\]
Hence, the concentration of \[N{O_2}\] and \[NO\] are\[\;0.4{\text{ }}mol/L\] and \[1.6{\text{ }}mol/L\]respectively.
Note: We can use equilibrium constant (Kc) to determine the extent of a reaction.therefore, the degree of the disappearance of the reactants. The magnitude of the equilibrium constant gives a knowledge of the relative amount of the reactants and the products in an equilibrium reaction.
\[Kc = \dfrac{{{{\left[ C \right]}^c}{{\left[ D \right]}^d}}}{{{{\left[ A \right]}^a}{{\left[ B \right]}^b}}}\]
Complete step by step solution:
We can define the equilibrium constant \[(Kc)\]is the ratio of the equilibrium concentrations of products upon the equilibrium concentrations of reactants and that each reactant and product raised to the power of their respective coefficients.
Thus,the equilibrium constant,\[Kc\] , for the reaction \[{\text{aA + bB }} \rightleftharpoons {\text{ cC + dD}}\] is given as,
\[Kc{\text{ }} = \]\[\dfrac{{{{\left[ C \right]}^c}{{\left[ D \right]}^d}}}{{{{\left[ A \right]}^a}{{\left[ B \right]}^b}}}\]
where A and B are reactants and C &D are products. a,b,c and d are their coefficients respectively.
Given,
Volume Of container = \[1{\text{ }}Liter\]
Equilibriumconstant = \[Kc{\text{ }} = {\text{ }}16\]
The equilibrium reaction is \[{\text{SO_2}}\left( {\text{g}} \right){\text{ + NO_2}}\left( {\text{g}} \right){\text{}} \rightleftharpoons {\text{SO_3}}\left( {\text{g}} \right){\text{ + NO}}\left( {\text{g}} \right){\text{}}\]
of each of the four gases given is \[1{\text{ }}M\]each,then
| \[S{O_2}\] | \[N{O_2}\] | \[S{O_3}\] | \[NO\] | |
| The initial concentrations | \[1M\] | \[1M\] | \[1M\] | 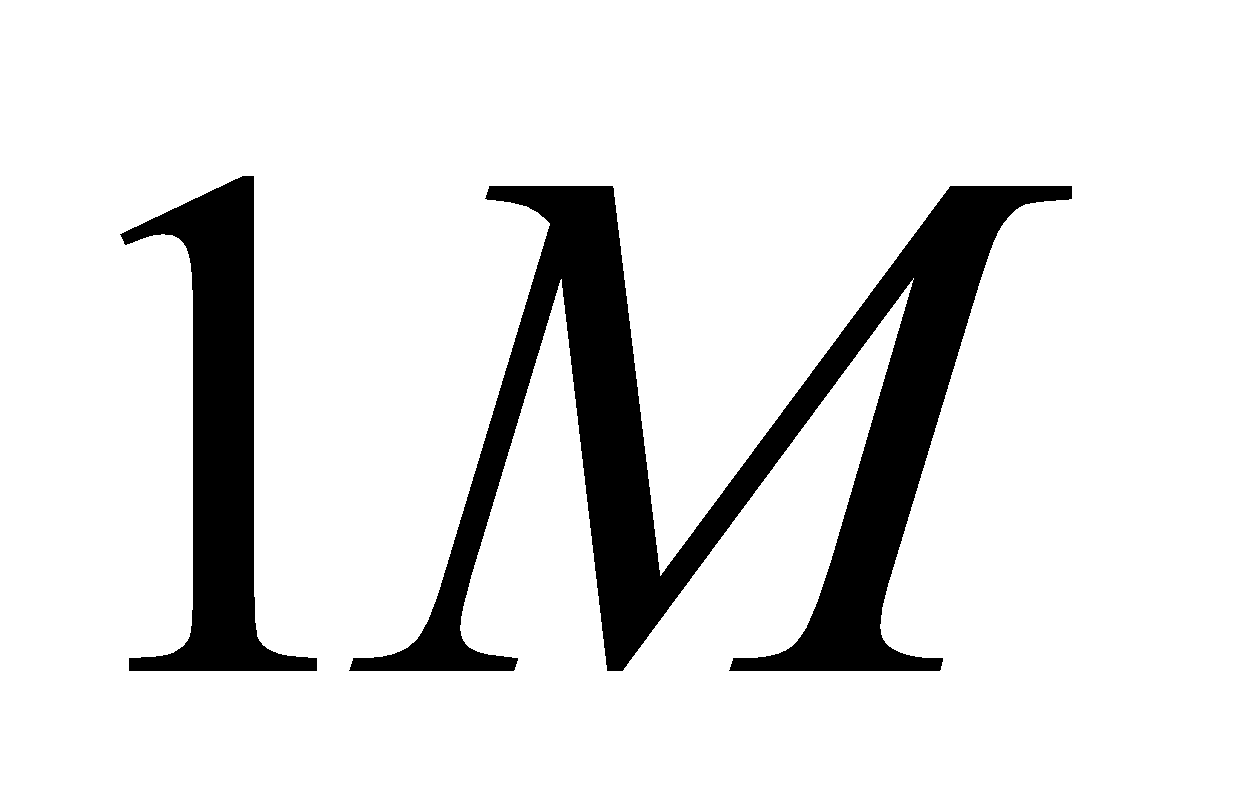
|
| The equilibrium concentrations | \[\left( {1 - x} \right)M\] | \[\left( {1 - x} \right)M\] | \[\left( {1 + x} \right)M\] | \[\left( {1 + x} \right)M\] |
Now, by applying the formula for the expression for the equilibrium constant,
\[Kc = \]\[\dfrac{{{{\left[ {SO_3} \right]}^{}}{{\left[ {NO} \right]}^{}}}}{{{{\left[ {SO_2} \right]}^{}}{{\left[ {NO_2} \right]}^{}}}}\]
Putting the values of equilibrium concentrations of reactants and products ,we get
\[Kc = \] \[\dfrac{{\left[ {1 + x} \right]\left[ {1 + x} \right]}}{{\left[ {1 - x} \right]\left[ {1 - x} \right]}}\] As \[Kc\] is 16 , then by solving the above we get,
∴ \[16 = \dfrac{{{{\left( {1 + x} \right)}^2}}}{{{{\left( {1 - x} \right)}^2}}}\]
∴ \[4 = \dfrac{{\left( {1 + x} \right)}}{{\left( {1 - x} \right)}}\] after simplifying above
∴ \[x{\text{ }} = {\text{ }}0.6\] By substituting the value of x ,
\[\left[ {S{O_3}} \right]{\text{ }} = {\text{ }}\left[ {NO} \right]{\text{ }} = {\text{ }}\left( {1 + {\text{ }}x} \right){\text{ }} = {\text{ }}1 + 0.6{\text{ }} = {\text{ }}1.6{\text{ }}mol/L\]
\[\left[ {S{O_2}} \right]{\text{ }} = {\text{ }}\left[ {N{O_2}} \right]{\text{ }} = {\text{ }}\left( {1 - {\text{ }}x} \right){\text{ }} = {\text{ }}1{\text{ }} - {\text{ }}0.6{\text{ }} = {\text{ }}0.4{\text{ }}mol/L\]
Hence, the concentration of \[N{O_2}\] and \[NO\] are\[\;0.4{\text{ }}mol/L\] and \[1.6{\text{ }}mol/L\]respectively.
Note: We can use equilibrium constant (Kc) to determine the extent of a reaction.therefore, the degree of the disappearance of the reactants. The magnitude of the equilibrium constant gives a knowledge of the relative amount of the reactants and the products in an equilibrium reaction.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

What is the difference between biodegradable and nonbiodegradable class 11 biology CBSE

Proton was discovered by A Thomson B Rutherford C Chadwick class 11 chemistry CBSE




