
Explain the industrial method of preparation of bleaching powder with a neat diagram.
Answer
604.2k+ views
Hint:Bleaching powder is a solid combination of chlorine (\[C{{l}_{2}}\]) and slaked lime (\[Ca{{\left( OH \right)}_{2}}\]). It was introduced in 1799 by Scottish chemist Charles Tennant. It is a pale yellow or nearly white colored inorganic powder. It smells like chlorine, i.e., pungent and irritating odor.
Complete step by step answer:
Chemically, bleaching powder is known as calcium hypochlorite or calcium oxychloride, with the chemical formula \[Ca{{\left( OCl \right)}_{2}}\].
Bleaching powder is prepared by passing chlorine gas (\[C{{l}_{2}}\]) over slaked lime (\[Ca{{\left( OH \right)}_{2}}\]).
\[Ca{{\left( OH \right)}_{2}}+C{{l}_{2}}\to Ca{{\left( OCl \right)}_{2}}+{{H}_{2}}O\]. The industrial preparation of bleaching powder is done through Hansenclever method.
Construction of Hasenclever Plant
Hasenclever plant consists of a number of hollow horizontal cylinders of iron, generally four in number. These cylinders are fitted with shafts having a large number of blades fixed along the entire length. These shafts can be rotated inside the cylinders which helps in stirring. Each of the cylinders are about 2m to 3m long. The uppermost cylinder has a hopper through which slaked lime is fed and an outlet for the waste gases to come out. The lowermost cylinder has an inlet through which chlorine gas is passed and an outlet for the final processed bleaching powder to come out.
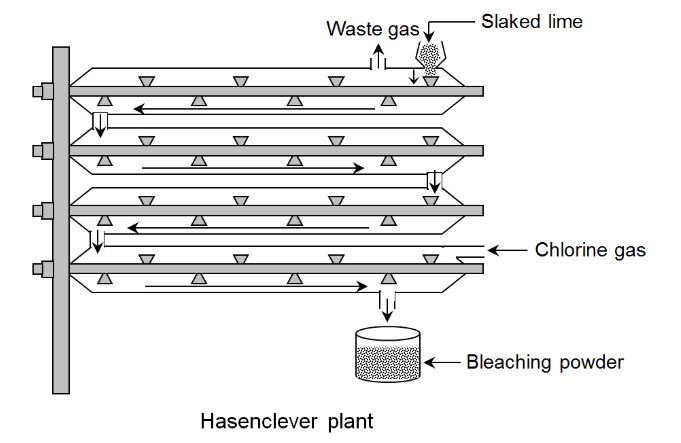
Working of Hasenclever Plant
First the dry slaked lime is fed into the Hasenclever plant through the hopper present in the uppermost cylinder. After that the dry slaked lime is moved forward by the revolving blades of the rotating shaft. Meanwhile, chlorine gas is passed through the inlet of the lowermost cylinder which rises up to the upper cylinders and reacts with the dry slaked lime coming downward to form bleaching powder. This bleaching powder comes out through the outlet at the bottom of the innermost cylinder and collected in a vessel.
Note:
Bleaching powder has wide applications in different industries. For example, it is used for bleaching wood pulp in the paper industry, bleaching cotton and linen in the textile industry, used for disinfecting drinking water supply and also used in the manufacturing of chloroform.
Complete step by step answer:
Chemically, bleaching powder is known as calcium hypochlorite or calcium oxychloride, with the chemical formula \[Ca{{\left( OCl \right)}_{2}}\].
Bleaching powder is prepared by passing chlorine gas (\[C{{l}_{2}}\]) over slaked lime (\[Ca{{\left( OH \right)}_{2}}\]).
\[Ca{{\left( OH \right)}_{2}}+C{{l}_{2}}\to Ca{{\left( OCl \right)}_{2}}+{{H}_{2}}O\]. The industrial preparation of bleaching powder is done through Hansenclever method.
Construction of Hasenclever Plant
Hasenclever plant consists of a number of hollow horizontal cylinders of iron, generally four in number. These cylinders are fitted with shafts having a large number of blades fixed along the entire length. These shafts can be rotated inside the cylinders which helps in stirring. Each of the cylinders are about 2m to 3m long. The uppermost cylinder has a hopper through which slaked lime is fed and an outlet for the waste gases to come out. The lowermost cylinder has an inlet through which chlorine gas is passed and an outlet for the final processed bleaching powder to come out.

Working of Hasenclever Plant
First the dry slaked lime is fed into the Hasenclever plant through the hopper present in the uppermost cylinder. After that the dry slaked lime is moved forward by the revolving blades of the rotating shaft. Meanwhile, chlorine gas is passed through the inlet of the lowermost cylinder which rises up to the upper cylinders and reacts with the dry slaked lime coming downward to form bleaching powder. This bleaching powder comes out through the outlet at the bottom of the innermost cylinder and collected in a vessel.
Note:
Bleaching powder has wide applications in different industries. For example, it is used for bleaching wood pulp in the paper industry, bleaching cotton and linen in the textile industry, used for disinfecting drinking water supply and also used in the manufacturing of chloroform.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




