
Draw the structure of the following compound:
p,p’-dihydroxybenzophenone
Answer
595.5k+ views
Hint:p,p’-dihydroxybenzophenone is an organic compound where two phenol groups are attached together with a carbonyl (-CO) group.
Complete step by step answer:
p,p’-dihydroxybenzophenone is a organic compound that have the molecular formula ${{\text{(HO}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}\text{)}}_{\text{2}}}\text{CO}$. As it names, there are two hydroxyl groups present in the para position (fourth position) in the phenyl ring counting from the central ketone group.
It produces by the rearrangement reaction of p-hydroxyphenyl benzoate which is as follows:
\[HO{{C}_{6}}{{H}_{4}}C{{O}_{2}}{{C}_{6}}{{H}_{5}}~\to \text{ }{{(HO{{C}_{6}}{{H}_{4}})}_{2}}CO\].
Alternatively, from p-hydroxybenzoic acid, p,p’-dihydroxybenzophenone can be formed. At first, p-hydroxybenzoic acid is converted to p-acetoxybenzoyl chloride. Further the acid chloride p-acetoxybenzoyl chloride upon deacetylation reaction with phenol generates 4,4'-dihydroxybenzophenone.
The structure of p,p’-dihydroxybenzophenone is drawn and as following:
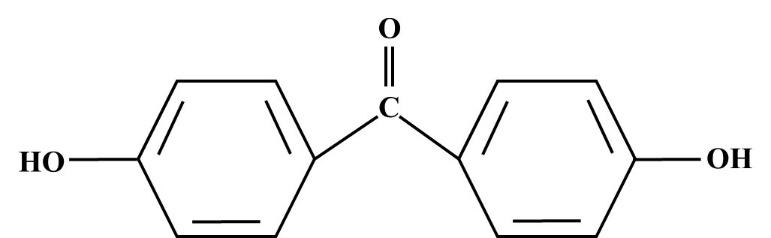
Additional information:The p,p’-dihydroxybenzophenone is a white solid product. It has many industrial application which are as follows:
-In fiber optical devices,
-In UV light stabilizers,
-In adhesives, cosmetics and plastic compounds,
-In electronic circuit boards,
-In polycarbonate polymers.
Note:
-p,p’-dihydroxybenzophenone is an organic compound that contains two phenol rings held together by a carbonyl group. The representative image is given in this solution above.
-In human physiology, the p,p’-dihydroxybenzophenone has adverse effects as it can act as an endocrine disruptor. In fungus, p,p’-dihydroxybenzophenone targets a particular enzyme Lanosterol 14-alpha demethylase. This particular enzyme catalyzes C14-demethylation of lanosterol that is an important agent for ergosterol biosynthesis. It converts lanosterol into 4,4'-dimethyl cholesta-8,14,24-triene-3-beta-ol. p,p’-dihydroxybenzophenone binds with the enzyme Lanosterol 14-alpha demethylase and blocks the lanosterol pathway. Thus by the above mechanism p,p’-dihydroxybenzophenone inhibits the fungal cell wall biosynthetic pathway and possesses anti-fungal activity.
Complete step by step answer:
p,p’-dihydroxybenzophenone is a organic compound that have the molecular formula ${{\text{(HO}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}\text{)}}_{\text{2}}}\text{CO}$. As it names, there are two hydroxyl groups present in the para position (fourth position) in the phenyl ring counting from the central ketone group.
It produces by the rearrangement reaction of p-hydroxyphenyl benzoate which is as follows:
\[HO{{C}_{6}}{{H}_{4}}C{{O}_{2}}{{C}_{6}}{{H}_{5}}~\to \text{ }{{(HO{{C}_{6}}{{H}_{4}})}_{2}}CO\].
Alternatively, from p-hydroxybenzoic acid, p,p’-dihydroxybenzophenone can be formed. At first, p-hydroxybenzoic acid is converted to p-acetoxybenzoyl chloride. Further the acid chloride p-acetoxybenzoyl chloride upon deacetylation reaction with phenol generates 4,4'-dihydroxybenzophenone.
The structure of p,p’-dihydroxybenzophenone is drawn and as following:

Additional information:The p,p’-dihydroxybenzophenone is a white solid product. It has many industrial application which are as follows:
-In fiber optical devices,
-In UV light stabilizers,
-In adhesives, cosmetics and plastic compounds,
-In electronic circuit boards,
-In polycarbonate polymers.
Note:
-p,p’-dihydroxybenzophenone is an organic compound that contains two phenol rings held together by a carbonyl group. The representative image is given in this solution above.
-In human physiology, the p,p’-dihydroxybenzophenone has adverse effects as it can act as an endocrine disruptor. In fungus, p,p’-dihydroxybenzophenone targets a particular enzyme Lanosterol 14-alpha demethylase. This particular enzyme catalyzes C14-demethylation of lanosterol that is an important agent for ergosterol biosynthesis. It converts lanosterol into 4,4'-dimethyl cholesta-8,14,24-triene-3-beta-ol. p,p’-dihydroxybenzophenone binds with the enzyme Lanosterol 14-alpha demethylase and blocks the lanosterol pathway. Thus by the above mechanism p,p’-dihydroxybenzophenone inhibits the fungal cell wall biosynthetic pathway and possesses anti-fungal activity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




