
Diamond is the hardest allotrope of carbon. Give reason for its hardness?
Answer
592.8k+ views
Hint: There are two allotropes for carbon. One allotrope is diamond and the other one is graphite. Among the allotropes of carbon diamond is hard in nature when compared to graphite because of the structure difference.
Complete answer:
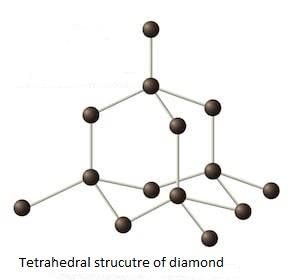
As we know that in diamond, the carbon atoms are arranged like a tetrahedral structure.
Diamond is strong, and has a rigid three-dimensional structure that results in an endless network of atoms.
This is the reason for diamond's hardness.
Due to the tetrahedral structure, diamond shows a great resistance to compression.
The hardness of the crystals is measured on a scale, developed by Friederich Mohs.
Diamond is the hardest material known till date (elected as 10 on the Mohs scale).
The Tetrahedral structure of the diamond is as follows.
Additional Information:
Diamond is the best conductor of heat.
It conducts up to five times the amount than that of copper.
Diamond also permits sound, but not electricity.
It is an insulator.
Its electrical resistance and chemical inertness are consistently remarkable.
Note: The carbon atoms in graphite structure are arranged in an endless array, but they are arranged in layers. The carbon atoms in graphite have two types of interactions. In the first type, each carbon atom is bonded to three other carbon atoms and the carbon atoms occupy the corners of a network of regular. Moreover these planar structures are held together by weak forces known as Van Der Waals forces.
Complete answer:
As we know that in diamond, the carbon atoms are arranged like a tetrahedral structure.
Diamond is strong, and has a rigid three-dimensional structure that results in an endless network of atoms.
This is the reason for diamond's hardness.
Due to the tetrahedral structure, diamond shows a great resistance to compression.
The hardness of the crystals is measured on a scale, developed by Friederich Mohs.
Diamond is the hardest material known till date (elected as 10 on the Mohs scale).
The Tetrahedral structure of the diamond is as follows.

Additional Information:
Diamond is the best conductor of heat.
It conducts up to five times the amount than that of copper.
Diamond also permits sound, but not electricity.
It is an insulator.
Its electrical resistance and chemical inertness are consistently remarkable.
Note: The carbon atoms in graphite structure are arranged in an endless array, but they are arranged in layers. The carbon atoms in graphite have two types of interactions. In the first type, each carbon atom is bonded to three other carbon atoms and the carbon atoms occupy the corners of a network of regular. Moreover these planar structures are held together by weak forces known as Van Der Waals forces.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




