
Define hydrogen bond. Explain different types of hydrogen bond giving examples .
Answer
529.7k+ views
Hint: We know that hydrogen bond is a weak force. When a hydrogen atom from one molecule is in close proximity with the electronegative atoms such as O,N,F etc., there is a development of electrostatic attraction.
Complete step by step solution:
As we know, hydrogen bonding is another most important binding force other than covalent and ionic bonds. It is another class of attractive forces that arise due to dipole-dipole interaction between a hydrogen atom that is bonded to a highly electronegative atom and another highly electronegative atom while lies in the vicinity of the hydrogen atom.
We can take one of the most common examples of hydrogen bonds are those formed in liquid alcohols. Most OH groups make a hydrogen bond to oxygen of an adjacent alcohol, thereby creating a network of hydrogen bonds. In liquid alcohols there is rapid interchange of the hydrogen bonds, with the molecules oriented imperfectly with their neighbors.
Thus, we can define hydrogen bonding as a very special class of intermolecular attractive forces that arise only in compounds which have hydrogen atoms bonded to a highly electronegative atom. Hydrogen bonds are mostly strong in comparison to normal dipole-dipole and dispersion forces. However, they are weak compared to true covalent or ionic bonds.
Types of Hydrogen bonding-
There are two types of H bonds, and the classification of it is mentioned below
1.Intermolecular Hydrogen bonding
2.Intramolecular Hydrogen bonding
1.Intermolecular Hydrogen bonding
When hydrogen bonding takes place between different molecules of the same or different compounds, it is called intermolecular hydrogen bonding.
For eg: hydrogen bonding in water, alcohol, ammonia etc
For better understanding, one diagrammatic representation is given below.
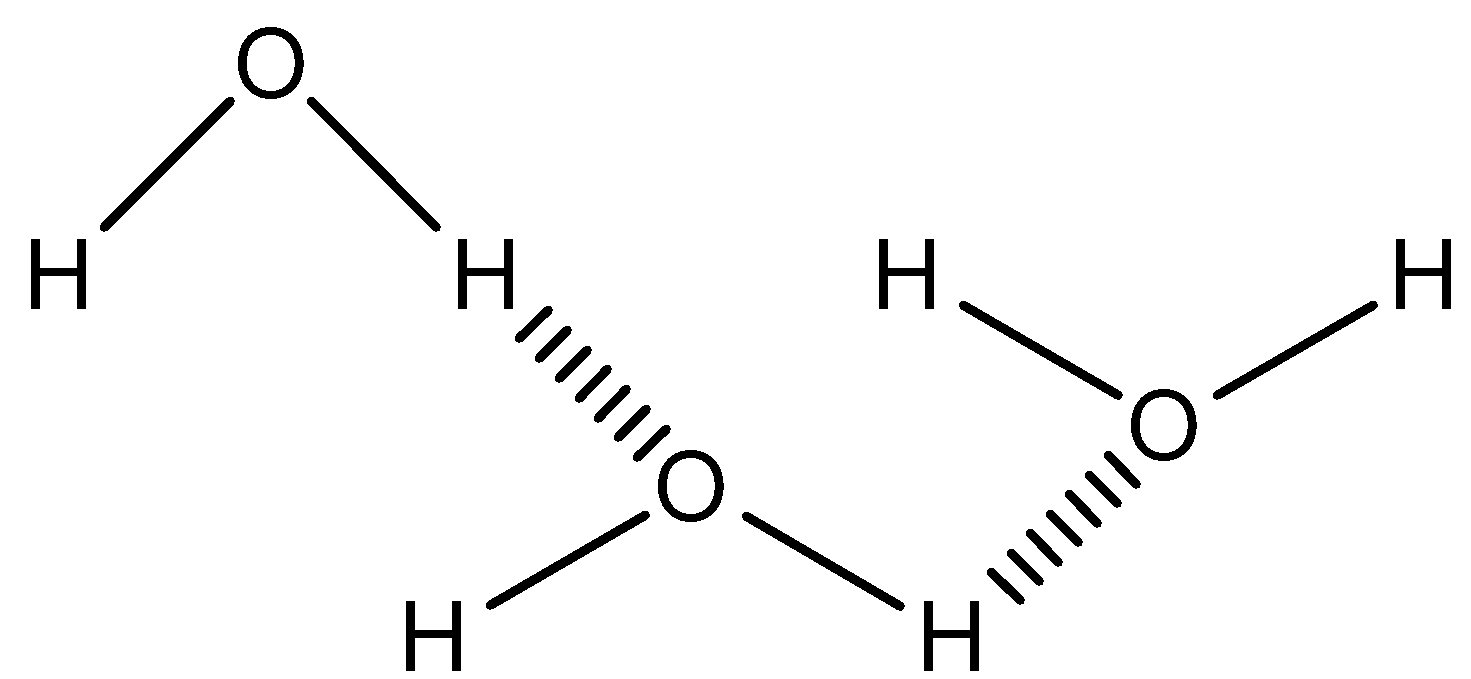
2.Intramolecular Hydrogen bonding
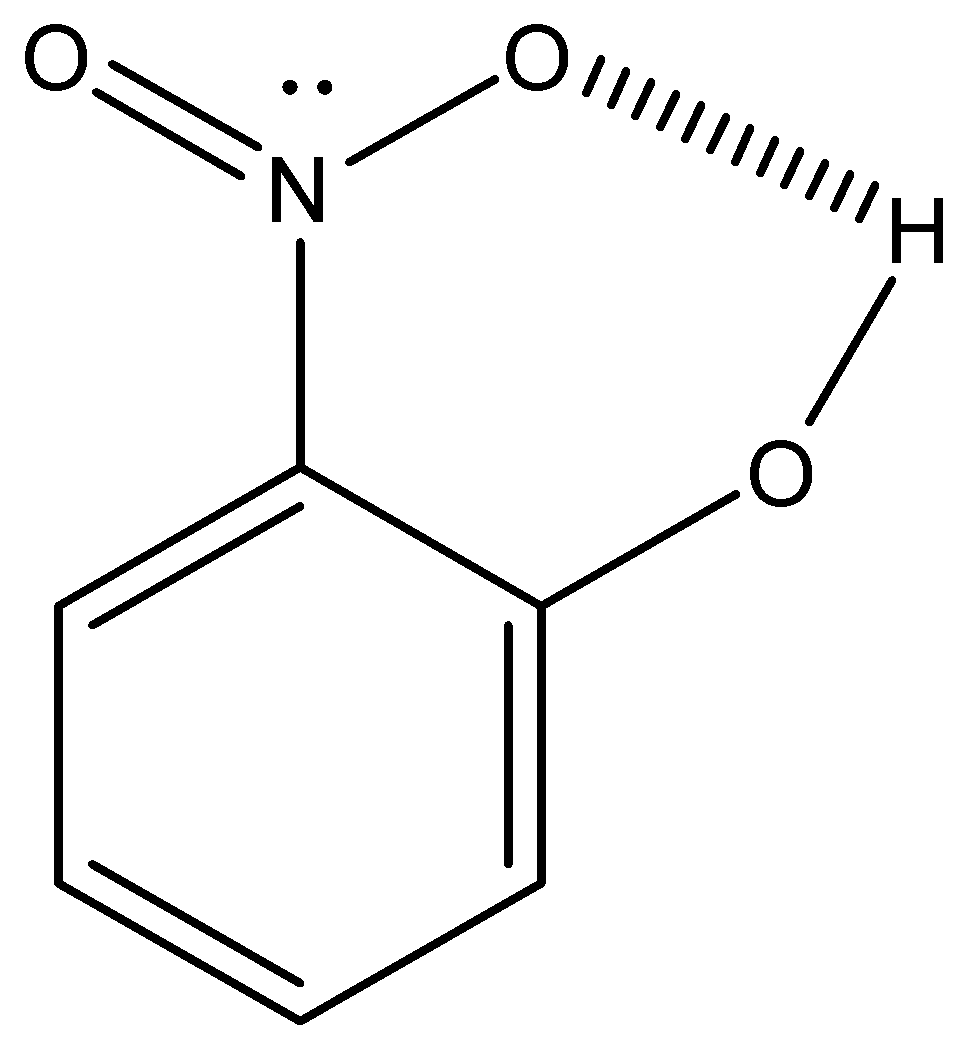
The hydrogen bonding which takes place within a molecule itself is called intramolecular hydrogen bonding. It takes place in compounds containing two groups such that one group contains a hydrogen atom linked to an electronegative atom and the other group contains a highly electronegative atom linked to a lesser electronegative atom of the other group within the same molecule.
The bond is formed between the hydrogen atoms of one group with the more electronegative atom of the other group within the same molecule.
For better understanding, one diagrammatic representation is given below.
Note: As the water molecule is shown in the example of intermolecular H-bonding, we should know about the number of hydrogen bonds a molecule can form. Each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds utilizing the hydrogen atoms attached to neighboring water molecules. These four hydrogen bonds optimally arrange themselves tetrahedrally around each water molecule as found in ice.
Complete step by step solution:
As we know, hydrogen bonding is another most important binding force other than covalent and ionic bonds. It is another class of attractive forces that arise due to dipole-dipole interaction between a hydrogen atom that is bonded to a highly electronegative atom and another highly electronegative atom while lies in the vicinity of the hydrogen atom.
We can take one of the most common examples of hydrogen bonds are those formed in liquid alcohols. Most OH groups make a hydrogen bond to oxygen of an adjacent alcohol, thereby creating a network of hydrogen bonds. In liquid alcohols there is rapid interchange of the hydrogen bonds, with the molecules oriented imperfectly with their neighbors.
Thus, we can define hydrogen bonding as a very special class of intermolecular attractive forces that arise only in compounds which have hydrogen atoms bonded to a highly electronegative atom. Hydrogen bonds are mostly strong in comparison to normal dipole-dipole and dispersion forces. However, they are weak compared to true covalent or ionic bonds.
Types of Hydrogen bonding-
There are two types of H bonds, and the classification of it is mentioned below
1.Intermolecular Hydrogen bonding
2.Intramolecular Hydrogen bonding
1.Intermolecular Hydrogen bonding
When hydrogen bonding takes place between different molecules of the same or different compounds, it is called intermolecular hydrogen bonding.
For eg: hydrogen bonding in water, alcohol, ammonia etc
For better understanding, one diagrammatic representation is given below.

2.Intramolecular Hydrogen bonding
The hydrogen bonding which takes place within a molecule itself is called intramolecular hydrogen bonding. It takes place in compounds containing two groups such that one group contains a hydrogen atom linked to an electronegative atom and the other group contains a highly electronegative atom linked to a lesser electronegative atom of the other group within the same molecule.
The bond is formed between the hydrogen atoms of one group with the more electronegative atom of the other group within the same molecule.
For better understanding, one diagrammatic representation is given below.

Note: As the water molecule is shown in the example of intermolecular H-bonding, we should know about the number of hydrogen bonds a molecule can form. Each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds utilizing the hydrogen atoms attached to neighboring water molecules. These four hydrogen bonds optimally arrange themselves tetrahedrally around each water molecule as found in ice.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




