
Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for this behaviour.

Answer
592.2k+ views
Hint: Type of hybridization in a molecule affects the electronegativity of the central atom. s-character of the hybrid orbitals is directly related to electronegativity. Increase in the electronegativity results in the development of partial charges on the atoms.
Complete answer:
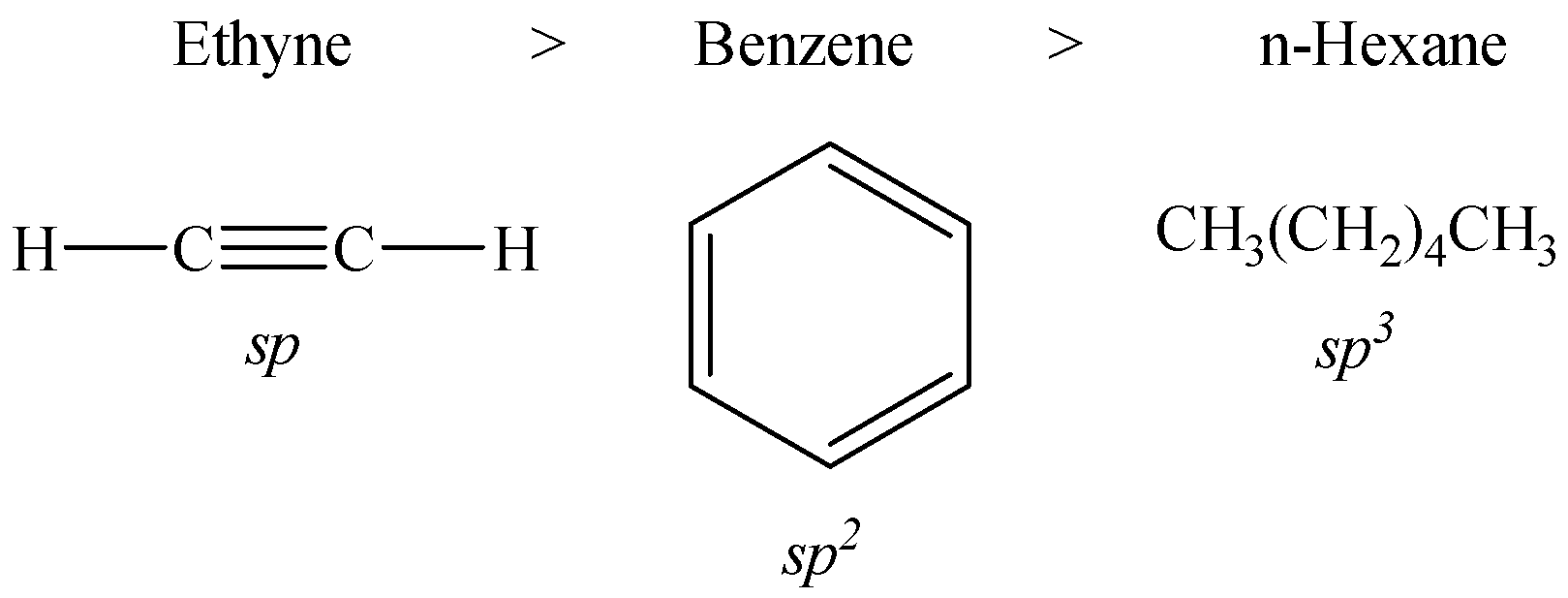
The decreasing order of acidic behaviour in benzene, n-hexane and ethyne is given below:
Let us now discuss the reason behind this behavior.
Acidic character of a molecule dictates the ability or ease of the molecule to give protons or ${{H}^{+}}$ ions.
During bond formation, atomic orbitals mix together to form new equivalent hybrid orbitals. Depending on the type orbitals undergoing hybridization, we have sp, $s{{p}^{2}}$, $s{{p}^{3}}$, etc. hybridization.
A sp-hybridized carbon atom has hybrid orbitals with 50% s-character. $s{{p}^{2}}$-hybridized carbon has 33.33% s-character in hybrid orbitals and s- character of $s{{p}^{3}}$-hybrid orbitals is 25% .
It is known that greater the s-character of the hybrid orbitals more is the electronegativity of the atom. It is due to the fact that s-orbitals are closer to the nucleus than p or d-orbitals. Thus, as the s-character in the orbital increases, the orbital lies closer and closer to the nucleus.
Therefore, as the s-character of the carbon atom in $C-H$ bond increases, the electron density in the s-orbital increases, i.e. the electrons of the bond lie more towards the carbon atom.
In other words, we can say that the electronegativity of the carbon atom increases since electronegativity is the ability of an atom to hold or attract electrons.
Due to this increase in electronegativity, there develops partial negative and positive charge on carbon and hydrogen atoms, respectively. As the positive charge on hydrogen increases, the acidic character increases.
The s-character of sp-hybridized carbon atom in ethyne, i.e. 50% is more than that of $s{{p}^{2}}$-hybridized carbon in benzene, i.e. 33.33% followed by $s{{p}^{3}}$-hybridized carbon in n-hexane with 25%.
Since s-character is directly proportional to electronegativity and electronegativity is related to acidic character, therefore, the acidic character in benzene, n-hexane and ethyne follows the above given order.
Note: Note here that although the number of s orbitals is same in all the three hybridizations, i.e. sp, $s{{p}^{2}}$ and $s{{p}^{3}}$, the number of p-orbitals or p-character is decreasing from $s{{p}^{3}}$ to sp. Hence, s-character is increasing $s{{p}^{3}}$ to sp.
Complete answer:
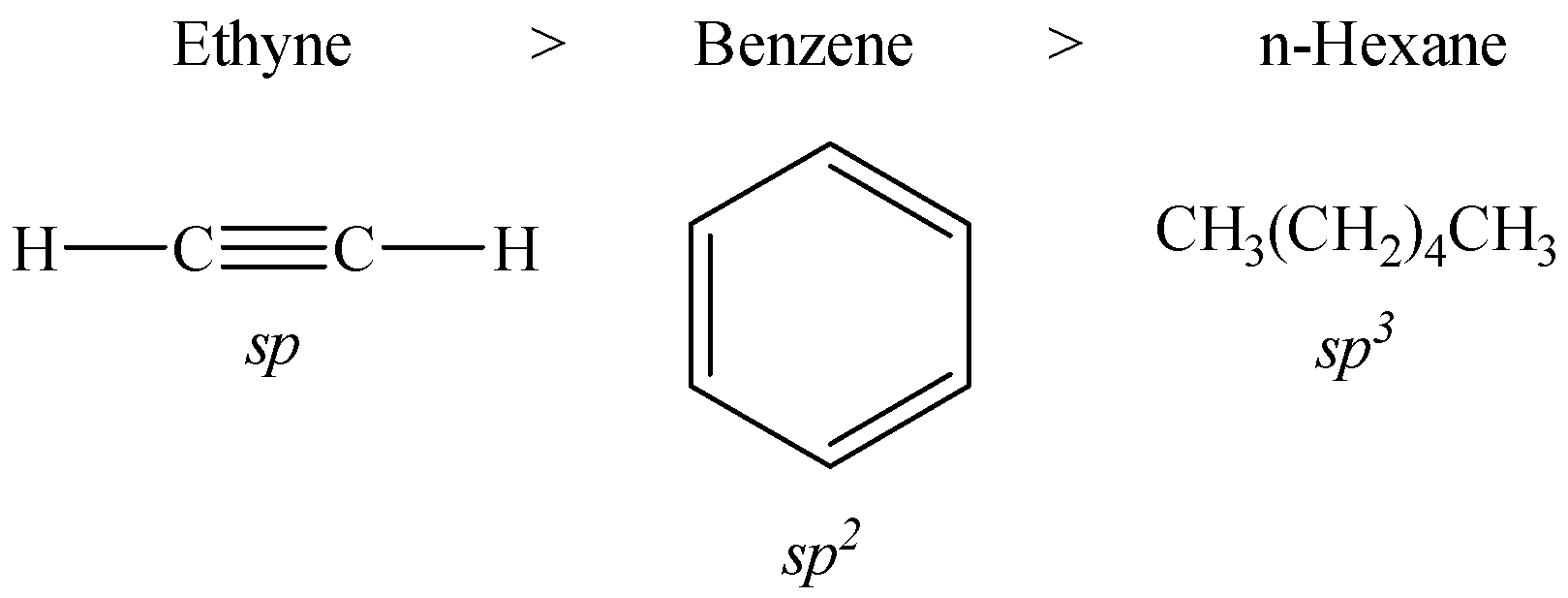
The decreasing order of acidic behaviour in benzene, n-hexane and ethyne is given below:

Let us now discuss the reason behind this behavior.
Acidic character of a molecule dictates the ability or ease of the molecule to give protons or ${{H}^{+}}$ ions.
During bond formation, atomic orbitals mix together to form new equivalent hybrid orbitals. Depending on the type orbitals undergoing hybridization, we have sp, $s{{p}^{2}}$, $s{{p}^{3}}$, etc. hybridization.
A sp-hybridized carbon atom has hybrid orbitals with 50% s-character. $s{{p}^{2}}$-hybridized carbon has 33.33% s-character in hybrid orbitals and s- character of $s{{p}^{3}}$-hybrid orbitals is 25% .
It is known that greater the s-character of the hybrid orbitals more is the electronegativity of the atom. It is due to the fact that s-orbitals are closer to the nucleus than p or d-orbitals. Thus, as the s-character in the orbital increases, the orbital lies closer and closer to the nucleus.
Therefore, as the s-character of the carbon atom in $C-H$ bond increases, the electron density in the s-orbital increases, i.e. the electrons of the bond lie more towards the carbon atom.
In other words, we can say that the electronegativity of the carbon atom increases since electronegativity is the ability of an atom to hold or attract electrons.
Due to this increase in electronegativity, there develops partial negative and positive charge on carbon and hydrogen atoms, respectively. As the positive charge on hydrogen increases, the acidic character increases.
The s-character of sp-hybridized carbon atom in ethyne, i.e. 50% is more than that of $s{{p}^{2}}$-hybridized carbon in benzene, i.e. 33.33% followed by $s{{p}^{3}}$-hybridized carbon in n-hexane with 25%.
Since s-character is directly proportional to electronegativity and electronegativity is related to acidic character, therefore, the acidic character in benzene, n-hexane and ethyne follows the above given order.
Note: Note here that although the number of s orbitals is same in all the three hybridizations, i.e. sp, $s{{p}^{2}}$ and $s{{p}^{3}}$, the number of p-orbitals or p-character is decreasing from $s{{p}^{3}}$ to sp. Hence, s-character is increasing $s{{p}^{3}}$ to sp.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




