
: An application of the Zeroth law of thermodynamics is
A.)Thermometer
B.)Barometer
C.)Manometer
D.)None of these
Answer
522.6k+ views
Hint: If A, B and C are three closed systems and A is in thermal equilibrium with B. Now C is separately in thermal equilibrium with B, then A and C must also be in thermal equilibrium.
Complete step by step solution
The Zeroth Law of thermodynamics is a definition for temperature, which can be used to measure thermal equilibriums.
No heat flows from A to B as they are in thermal equilibrium.
.
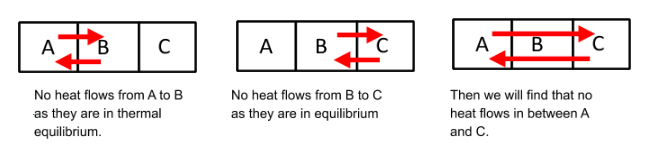
We know measure of temperature helps us to determine whether heat will flow from one object to another. Using this law we can determine and compare the temperatures of different objects.
Thermometers are the most elegant example for the First Law of thermodynamics in action. Thermometers have a thin column of mercury within a glass casing. When the temperature of mercury increases, it expands within the tube. Since the area of the tube is fixed, the expansion causes a rise in height of the mercury column, which is interpreted as rise in temperature.
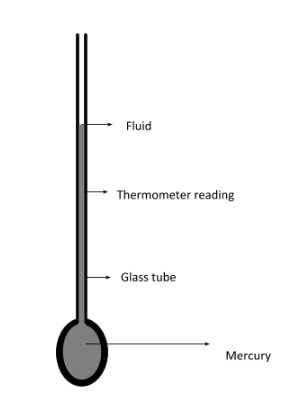
When a thermometer is used to measure our body temperature the glass wall attains thermal equilibrium with our body. And the mercury which is in contact with the glass wall will eventually reach thermal equilibrium. Using the zeroth law of thermodynamics we can say that our body and mercury are in thermal equilibrium i.e. in same temperature.This is how mercury thermometer measures the temperature.
Note :
Though a thermometer needs to be in contact with the system to measure temperature, Physical contact is not always necessary for heat to flow. There are various modes of heat transfer, such as conduction, convection and radiation.
Barometer is a device used to measure air pressure and a manometer is used to measure gas pressure. Thus, these two options do not involve the application of Zeroth Law.
Complete step by step solution
The Zeroth Law of thermodynamics is a definition for temperature, which can be used to measure thermal equilibriums.
No heat flows from A to B as they are in thermal equilibrium.
.

We know measure of temperature helps us to determine whether heat will flow from one object to another. Using this law we can determine and compare the temperatures of different objects.
Thermometers are the most elegant example for the First Law of thermodynamics in action. Thermometers have a thin column of mercury within a glass casing. When the temperature of mercury increases, it expands within the tube. Since the area of the tube is fixed, the expansion causes a rise in height of the mercury column, which is interpreted as rise in temperature.

When a thermometer is used to measure our body temperature the glass wall attains thermal equilibrium with our body. And the mercury which is in contact with the glass wall will eventually reach thermal equilibrium. Using the zeroth law of thermodynamics we can say that our body and mercury are in thermal equilibrium i.e. in same temperature.This is how mercury thermometer measures the temperature.
Note :
Though a thermometer needs to be in contact with the system to measure temperature, Physical contact is not always necessary for heat to flow. There are various modes of heat transfer, such as conduction, convection and radiation.
Barometer is a device used to measure air pressure and a manometer is used to measure gas pressure. Thus, these two options do not involve the application of Zeroth Law.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




