
All kinds of matter is made up of extremely small particles, called_____________.
(A) atom
(B) molecule
(C) ion
(D) compound
Answer
600.6k+ views
Hint: Democritus named the small particles. He derived the name from the Greek word ‘atomos’ that means indivisible. John Dalton, father of ‘atomic theory’ reintroduced the particles. Then, Rutherford did the Gold-foil experiment and concluded that the unit of matter consists of three further sub-particles namely electrons, protons and neutrons.
Complete step by step answer:
- All kinds of matter are composed of small particles, called atoms.
- Atoms are the unit of matter. All living and nonliving things are composed of atoms.
- The centre of the atom consists of positively charged protons and neutral neutrons and makes up for the weight of an atom.
- The negatively charged electrons residing in various stationary orbitals cancel out the positive charge present in the centre of the nucleus.
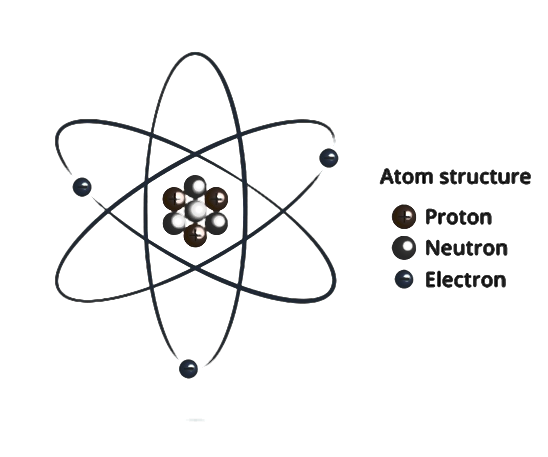
- Atoms are essential because they make up all materials on the earth. These are bound together by nuclear force and bond to form molecules which again form matter.
- Atoms cannot be formed nor destroyed. This statement is valid based on the law of conservation of mass.
- Electric force holds atoms together and the nuclear force holds the nucleus together.
So, the correct option is A.
Note: Space may seem to be empty, but it is not. It is composed of atoms. The size of the atom is \[{{10}^{-10}}\]metres.
Protons and electrons are of the same masses and equal and opposite charges. Protons and neutrons are together called nucleons. The number of protons in the nucleus is called the atomic number. The total number of protons and neutrons is called mass number.
Complete step by step answer:
- All kinds of matter are composed of small particles, called atoms.
- Atoms are the unit of matter. All living and nonliving things are composed of atoms.
- The centre of the atom consists of positively charged protons and neutral neutrons and makes up for the weight of an atom.
- The negatively charged electrons residing in various stationary orbitals cancel out the positive charge present in the centre of the nucleus.

- Atoms are essential because they make up all materials on the earth. These are bound together by nuclear force and bond to form molecules which again form matter.
- Atoms cannot be formed nor destroyed. This statement is valid based on the law of conservation of mass.
- Electric force holds atoms together and the nuclear force holds the nucleus together.
So, the correct option is A.
Note: Space may seem to be empty, but it is not. It is composed of atoms. The size of the atom is \[{{10}^{-10}}\]metres.
Protons and electrons are of the same masses and equal and opposite charges. Protons and neutrons are together called nucleons. The number of protons in the nucleus is called the atomic number. The total number of protons and neutrons is called mass number.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Who is eligible for RTE class 9 social science CBSE

What is the Full Form of ISI and RAW

How do you find the valency of chlorine sulphur and class 9 chemistry CBSE

What are the major achievements of the UNO class 9 social science CBSE

Explain the importance of pH in everyday life class 9 chemistry CBSE

Differentiate between parenchyma collenchyma and sclerenchyma class 9 biology CBSE




