
Acetone can be converted into pinacol by:
(a) Mg/Hg/${ H }_{ 2 }O$
(b) Zn/Hg/HCl
(c) Na/Hg/${ H }_{ 2 }{ SO }_{ 4 }$
(d) all of the above
Answer
581.7k+ views
Hint: Pinacol is a vicinal diol. For the pinacol formation from acetone we need an electron donor since it is a free radical reaction. This reaction is named after pinacol (2,3-Dimethyl-2,3-butanediol) which is the product that we get when the ketone used is acetone.
Complete step by step answer:
When ketones are reduced with a magnesium amalgam or zinc amalgam or sodium amalgam followed by treatment with water or a mild acid, we get vicinal diols. The mechanism for such a reaction is free-radical reaction.
${ CH }_{ 3 }-C(O)-{ CH }_{ 3 }\xrightarrow [{ H }_{ 3 }{ O }^{ + } ]{ Metal\quad amalgam } { (CH }_{ 3 }{ ) }_{ 2 }-C(OH)-C(OH)-{ (CH }_{ 3 }{ ) }_{ 2 }$
Where the metal amalgam could be sodium amalgam, magnesium amalgam or zinc amalgam.
We will show the mechanism for the pinacol formation using magnesium amalgam. The mechanism is similar if other metal amalgams are used.
The first step involves the reduction of the carbonyl group of acetone by the magnesium metal in order to form a ketyl radical anion species. The reaction is shown below:
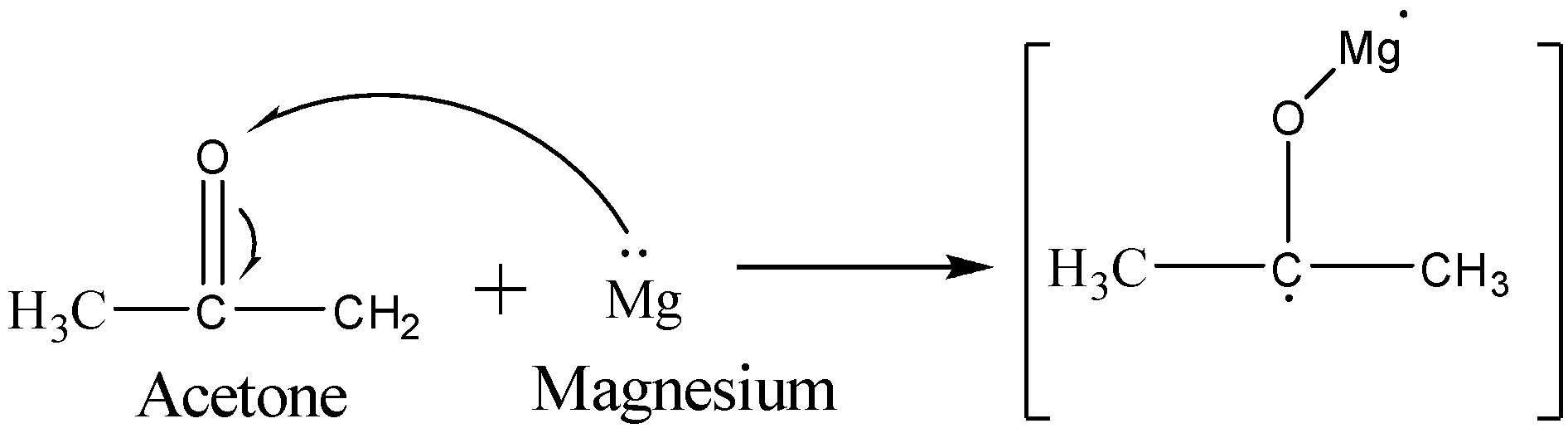
Now, one more molecule of acetone reacts with the magnesium metal in order to form ketyl radical anion species. The two ketyl radical anion species will react with each other to give a coupling reaction to give a vicinal diol precursor. The reaction is shown below:
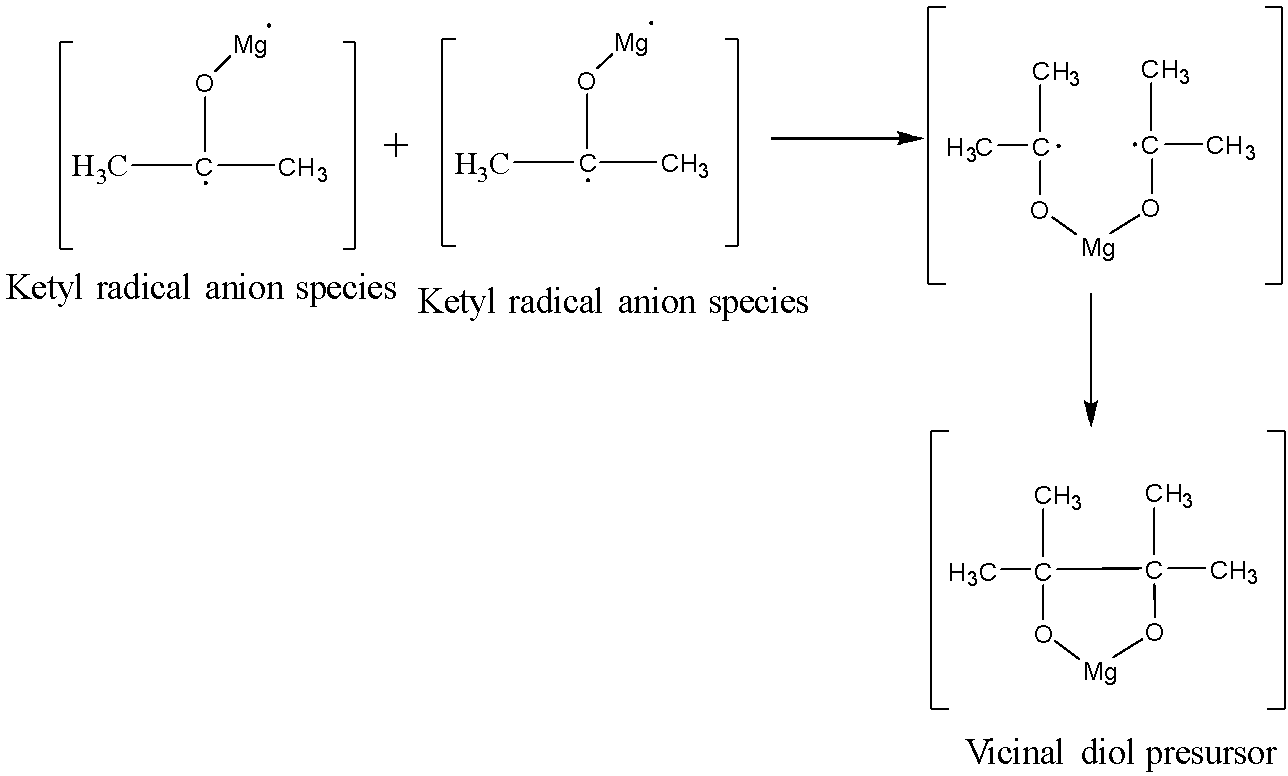
The vicinal diol precursor on acidification will give the vicinal diol. Actually on acidification, the five membered ring in the vicinal diol precursor breaks in order to form magnesium hydroxide and the pinacol.
The reaction is given below:
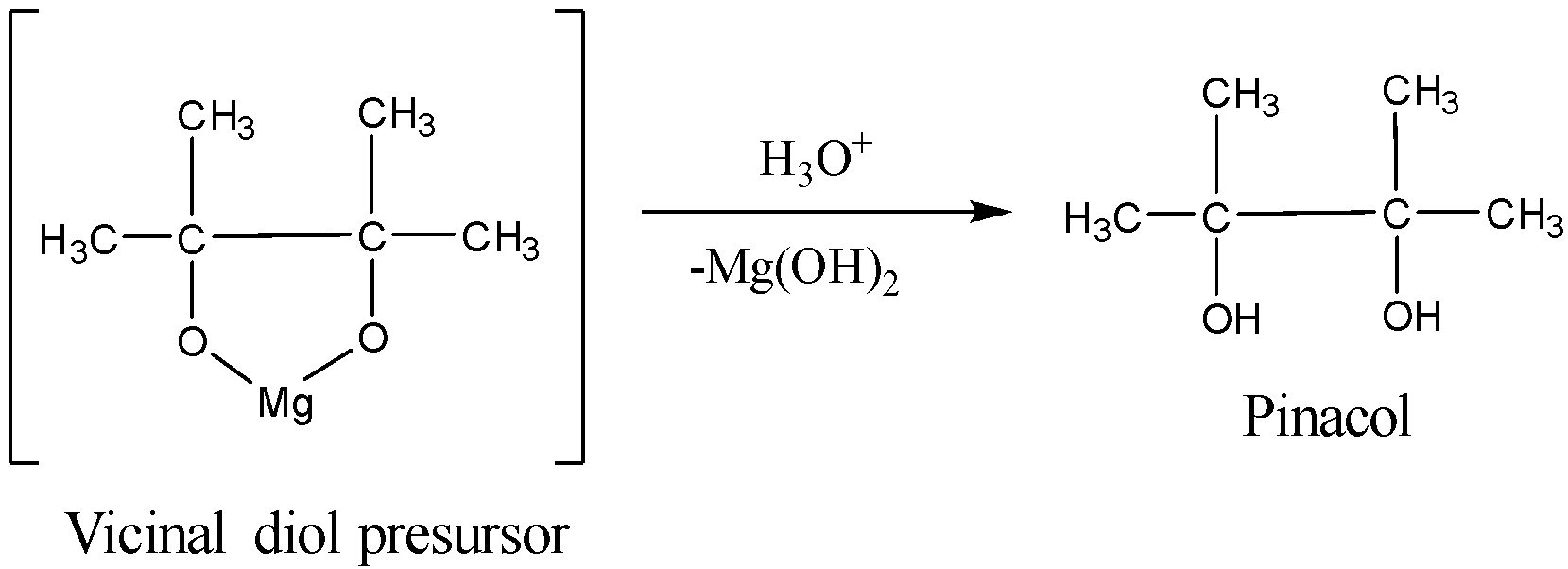
So, the correct answer is “Option D”.
Note: When pinacol is heated with concentrated sulphuric acid, it undergoes dehydration followed by migration of a methyl group to the adjacent carbon in order to form tert-butyl methyl ketone which is called pinacolone. This reaction is also shown by other substituted vicinal diols and is called pinacol-pinacolone rearrangement.
Complete step by step answer:
When ketones are reduced with a magnesium amalgam or zinc amalgam or sodium amalgam followed by treatment with water or a mild acid, we get vicinal diols. The mechanism for such a reaction is free-radical reaction.
${ CH }_{ 3 }-C(O)-{ CH }_{ 3 }\xrightarrow [{ H }_{ 3 }{ O }^{ + } ]{ Metal\quad amalgam } { (CH }_{ 3 }{ ) }_{ 2 }-C(OH)-C(OH)-{ (CH }_{ 3 }{ ) }_{ 2 }$
Where the metal amalgam could be sodium amalgam, magnesium amalgam or zinc amalgam.
We will show the mechanism for the pinacol formation using magnesium amalgam. The mechanism is similar if other metal amalgams are used.
The first step involves the reduction of the carbonyl group of acetone by the magnesium metal in order to form a ketyl radical anion species. The reaction is shown below:

Now, one more molecule of acetone reacts with the magnesium metal in order to form ketyl radical anion species. The two ketyl radical anion species will react with each other to give a coupling reaction to give a vicinal diol precursor. The reaction is shown below:

The vicinal diol precursor on acidification will give the vicinal diol. Actually on acidification, the five membered ring in the vicinal diol precursor breaks in order to form magnesium hydroxide and the pinacol.
The reaction is given below:

So, the correct answer is “Option D”.
Note: When pinacol is heated with concentrated sulphuric acid, it undergoes dehydration followed by migration of a methyl group to the adjacent carbon in order to form tert-butyl methyl ketone which is called pinacolone. This reaction is also shown by other substituted vicinal diols and is called pinacol-pinacolone rearrangement.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




