




Introduction to Surface Chemistry
Surface chemistry is concerned with events that occur at the surfaces or interfaces of materials. By separating the bulk phases by a surface, the interface or surface is represented. A hyphen or a slash can be used. The interface between, for example, Solid-gas can indicate both a solid and a gas. Alternatively, solid/gas There is no such thing as total miscibility. We may come across pure surface chemistry, solutions or compounds. In most cases, the interface is only a few molecules thick, but its area is proportional to its size of the bulk phase's particles. Among these are a variety of phenomena, the most notable of which is electrode processes, corrosion, heterogeneous at the same time, catalysis, dissolution, and crystallisation occur. Surface chemistry is a topic that has a wide range of applications.
Important Topics of Surface Chemistry
Adsorption
Colloids
Emulsions
Catalysis
Classification of Colloids
Colloids Around Us
1. Adsorption
There are various instances that show how a solid's surface has a tendency to attract and keep the molecules of the phase with which it interacts. When it comes into contact These chemicals are only found on the surface and do not penetrate deep into the body.
The term "surface" refers to what happens on the surface of a solid or liquid rather than in the bulk of it.
The molecular species or substance that concentrates oror accumulates at the surface is called the adsorbate and the material on the surface of which the adsorption takes place is called adsorbent.
The adsorbent is the surface on which the adsorption takes place.
Adsorption is a phenomenon that occurs on the surface of a substance. Solids, specifically charcoal, in its finely split state, has a vast surface area.
(a) - Distinction between Adsorption and Absorption
The material is concentrated exclusively at the surface of the adsorbent and does not pass through the surface to the bulk of the adsorbent in adsorption. The material is consistently dispersed during absorption in most of the solid. When a chalk stick is dipped in ink then because of the adsorption of coloured particles, the surface preserves the ink's colour. When the ink's solvent penetrates deeper into the stick owing to be absorbed When you break the chalk stick, we will see that it's white inside. There is a difference between absorption and adsorption.
Using water vapour as an example, vapours of water are absorbed by Calcium chloride, anhydrous, but adsorbed by silica gel.
(b) - Mechanism of Adsorption
Adsorption occurs when the adsorbent's surface particles are not in the same environment as the particles within the bulk and all forces acting between the particles in the adsorbent are mutual.
Although the particles are balanced, they are not surrounded by atoms on the surface. On all sides, they are surrounded by molecules of their sort, and as a result, they are unbalanced. Alternatively, leftover attractive forces. The adsorbent's forces are liable for the purpose of attracting adsorbate particles to its surface
The magnitude of Adsorption increases as the surface area per unit mass of the substance increases at a particular temperature and pressure.
In a spontaneous adsorption process, the combination of these two elements causes ∆G to be negative. As the adsorption process progresses, H becomes less and less negative until it equals T∆S and ∆G is zero. At this point, equilibrium has been achieved.
(c) - Types of Adsorption
There are primarily two forms of gas adsorption on solids-
Physical adsorption, also known as physisorption, happens when a gas accumulates on the surface of a solid due to weak van der Waals' forces.
Chemical adsorption, also known as chemisorption, occurs when gas molecules or atoms are held to a solid surface by chemical bonds.
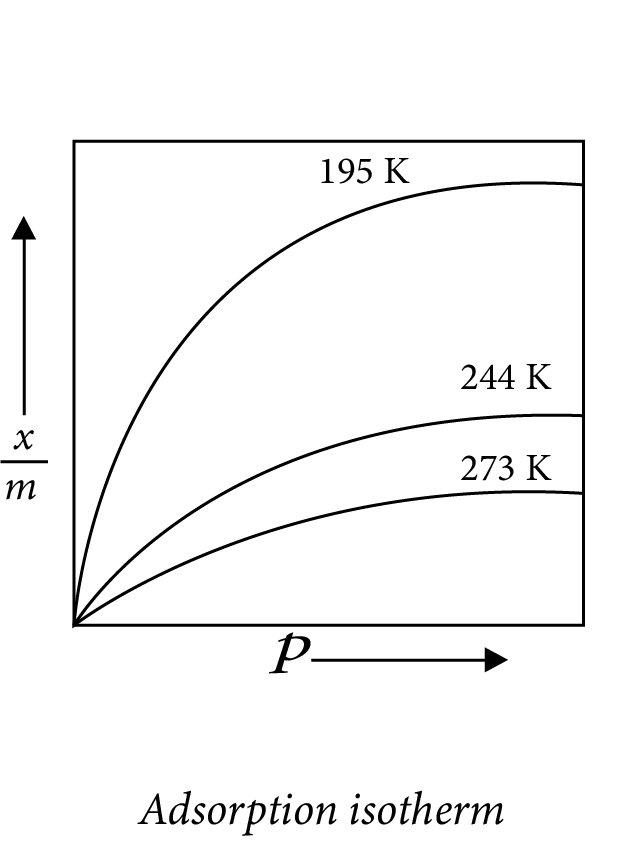
(d) - Adsorption Isotherms
The adsorption isotherm is a curve that depicts the variation in the amount of gas adsorbed by the adsorbent as a function of pressure at constant temperature.
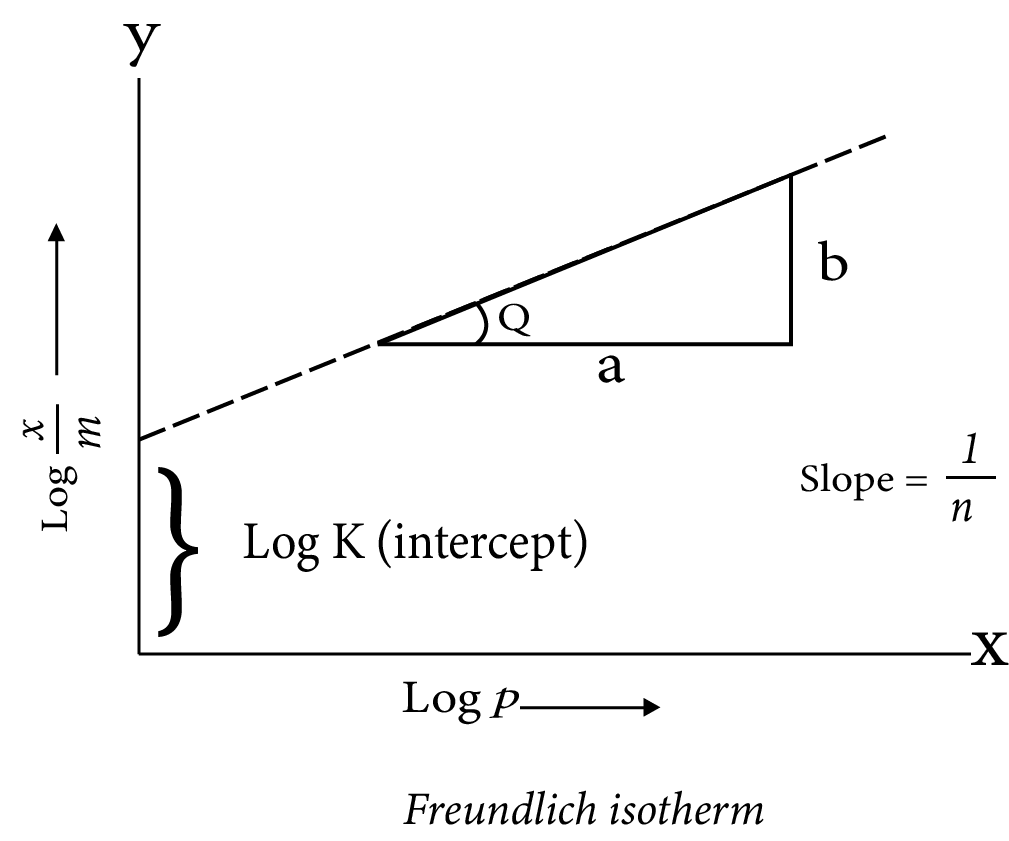
Isotherm of Freundlich adsorption: Freundlich established an empirical relationship between the amount of gas adsorbed per unit mass of solid adsorbent and pressure at a given temperature in 1909. The following equation can be used to express the relationship:
x/m = k.p 1/n (n > 1)
where x is the mass of the gas adsorbed on mass m of the adsorbent at pressure P, k and n are the constants which depend on the nature of the adsorbent and the gas at a particular temperature.
Taking logarithm
log x/m = log k + 1/n log p
The validity of Freundlich isotherm can be verified by plotting log x m on y-axis and log p on x-axis . If it comes to be a straight line, the Freundlich isotherm is valid, otherwise not (Fig. 5.2). The slope of the straight line gives the value of 1 n . The intercept on the y-axis gives the value of log k.
(e) - Applications of Adsorption
The adsorption phenomenon has a wide range of uses, the following are some of the most important:
High-vacuum production: The last vestiges of air can be adsorbed by charcoal from a vessel that has been evacuated by a vacuum pump, resulting in a very high vacuum.
Gas masks: In coal mines, gas masks (a device made of activated charcoal or a mixture of adsorbents) are commonly used to adsorb dangerous gases.
Humidity control: Adsorbents such as silica and aluminium gels are used to remove moisture and control humidity.
Colour removal from solutions: Animal charcoal adsorbs coloured contaminants to remove colour from solutions.
Inert gas separation: A mixture of noble gases can be separated by the difference in degree of adsorption of gases by charcoal. At various temperatures, adsorption on coconut charcoal was tested.
In the treatment of diseases, a variety of medications are utilised to kill germs.
The froth floatation technique concentrates a low-grade sulphide ore by using this process to separate it from silica and other earthy substances, a foaming agent and pine oil.
2. Catalysis
Berzelius was the first to do a comprehensive investigation of the effect of numerous foreign compounds on the speeds of chemical reactions, in 1835. He coined the term "catalyst" to describe such compounds.
Catalysts are substances that speed up the rate of a chemical process while remaining chemically and quantitatively unaltered afterward, and the phenomenon is known as catalysis.
(a) - Homogeneous and Heterogeneous Catalysis
Homogeneous catalysis occurs when the reactants, their products, and the catalyst are all in the same phase (liquid or gas). For example, In the lead chamber method, oxidation of sulphur dioxide to sulphur trioxide with dioxygen in the presence of nitrogen oxides as the catalyst. Sulphur dioxide and oxygen, as well as nitric oxide, the catalyst, are all in the same phase.
Heterogeneous catalysis is a catalytic process that occurs when the reactants and catalysts are in different phases. For example, In the presence of Pt, sulphur dioxide is oxidised to sulphur trioxide, in this the reactant is in a gaseous form, the catalyst is solid.
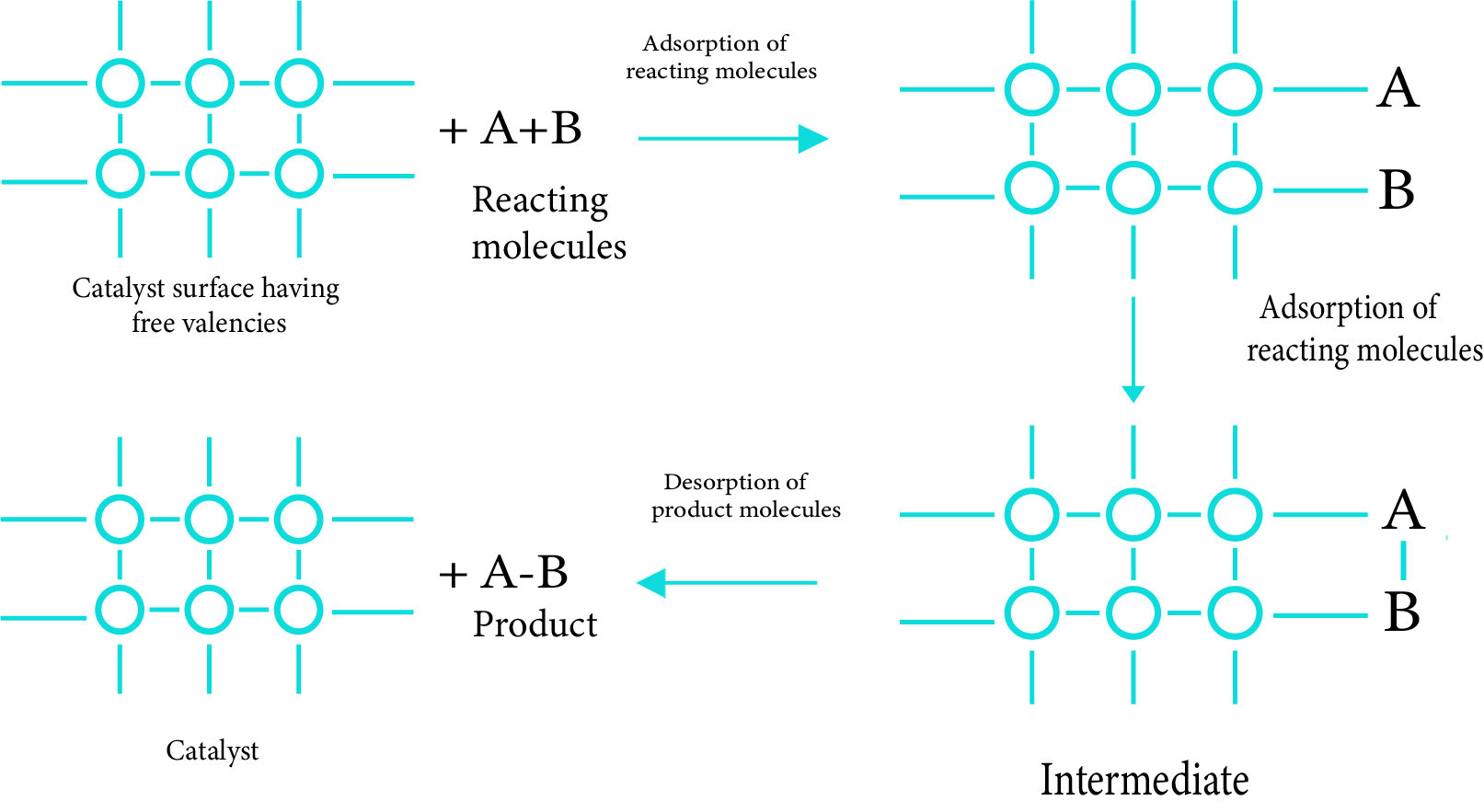
(b) - Adsorption Theory of Heterogeneous Catalysis
The classical adsorption theory and the intermediate compound production theory are combined in the current adsorption theory. The catalytic activity is concentrated on the catalyst's surface. There are five steps to the mechanism:
Diffusion of reactants to the catalyst's surface.
Reactant molecule adsorption on the catalyst's surface.
The occurrence of a chemical reaction on the catalyst's surface, as evidenced by the creation of an intermediate.
Desorption of reaction products from the catalyst surface, allowing the surface to be used for other reactions.
Reaction product diffusion away from the catalyst's surface.
Important Features of Solid Catalysts
(a) Activity
To a considerable part, a catalyst's activity is determined by the strength of chemisorption. The reactants must be adsorbed in a reasonable amount of time firmly on the catalyst in order for it to become active. They must, nevertheless, not be immobilised by the adsorptive force of the adsorptive force of the adsorptive force of the adsorptive force of the adsorption. There is no room on the catalyst's surface for reactants.
(b) Selectivity
The selectivity of a catalyst is its ability to direct a reaction to yield a particular product selectively, when under the same reaction conditions many products are possible. Selectivity of different catalysts for the same reactants is different.
(c) - Shape Selective Catalysis by Zeolites
Shape-selective catalysis is a catalytic process that is influenced by the pore structure of the catalyst as well as the size of the reactant and product molecules. Because of their honeycomb-like architecture, zeolites are good shape-selective catalysts. They have microporous surfaces; aluminosilicates are three-dimensional silicate networks in which certain silicon atoms are replaced with aluminium atoms, resulting in aluminosilicates. Framework made of Al–O–Si. The reactions that occur in zeolites are influenced by a variety of factors like the size and form of the reactant and product molecules, as well as the size and shape of the reactant and product molecules the zeolites' pores and cavities They can also be found in nature and used for catalytic selectivity as synthesised. Zeolites are commonly employed as catalysts in the petrochemical industry and also Hydrocarbon cracking and isomerization industries. A key consideration ZSM-5 is a zeolite catalyst used in the petroleum sector as the alcohols are converted directly into gasoline (petrol) by dehydrating them.
(d) - Enzyme Catalysis
Enzyme catalysis is unique in its efficiency and has a high degree of specificity. The following characteristics are shown by enzyme catalysts:
Highest Efficiency: One enzyme molecule may convert one million molecules of the reactant per minute.
Highly Specific Nature: Each enzyme catalyses only one reaction, hence a single catalyst cannot catalyse several reactions. The enzyme urease, for example, only catalyses the hydrolysis of urea. Any other amide hydrolysis is not catalysed by it.
Highly Ative at Optimal Temperature: An enzyme's rate of reaction reaches its maximum at a specific temperature, known as the optimum temperature. The enzyme activity diminishes on either side of the optimal temperature.
3. Colloids
A colloid is a heterogeneous system in which one component is scattered as very small particles in another material called the dispersion medium (dispersed phase).
The primary distinction between a solution and a colloid is particle size. In a colloid, the dispersed phase may consist of particles of a single macromolecule (such as protein or synthetic polymer) or an aggregate of many atoms, ions, or molecules, whereas in a solution, the constituent particles are ions or tiny molecules. Colloidal particles are larger than simple molecules yet still small enough to float. Their sizes range from 1 to 1000 nm.
4. Classification of Colloids of Colloids
The following criteria are used to categorise colloids:
Physical state of dispersed phase and dispersion medium
Nature of dispersed phase-dispersion medium interaction
Dispersed phase particle type.
(a) - Classification Based on Physical State of Dispersed Phase and Dispersion Medium
There are eight different types of colloidal systems depending on whether the dispersed phase and the dispersion medium are solids, liquids, or gases.
(b) - Classification Based on Nature of Interaction between Dispersed Phase and Dispersion Medium
Lyophilic Colloids: Lyophilic colloids are liquid-loving. Lyophilic sols are colloidal sols made by combining substances such as gum, gelatine, starch, rubber, and others with a suitable liquid (the dispersion medium). The fact that if the dispersion media is detached from the dispersed phase (for example, by evaporation), the sol may be rebuilt by simply mixing it with the dispersion medium is a key feature of these sols.
Lyophobic Colloids: The term "lyophobic" refers to a substance that dislikes liquid.
Metals, their sulphides, and other substances do not form colloidal sol when merely mixed with the dispersion medium.
(c) - Classification Based on Type of Particles of the Dispersed Phase, Multimolecular, Macromolecular and Associated Colloids
Multimolecular colloids: When a substance dissolves, a large number of atoms or smaller molecules produce a colloidal structure, species with a colloidal size range of 1–1000 nm.A good example is a particles of varied sizes with numerous atoms may be found in gold sol.
Macromolecular Colloids: These are in the suitable solvents form solutions in which the size of the macromolecules may be in the colloidal range. Such systems are called macromolecular colloids. Example, starch, cellulose, proteins and enzymes; and those of man-made macromolecules are polythene, nylon, polystyrene, synthetic rubber.
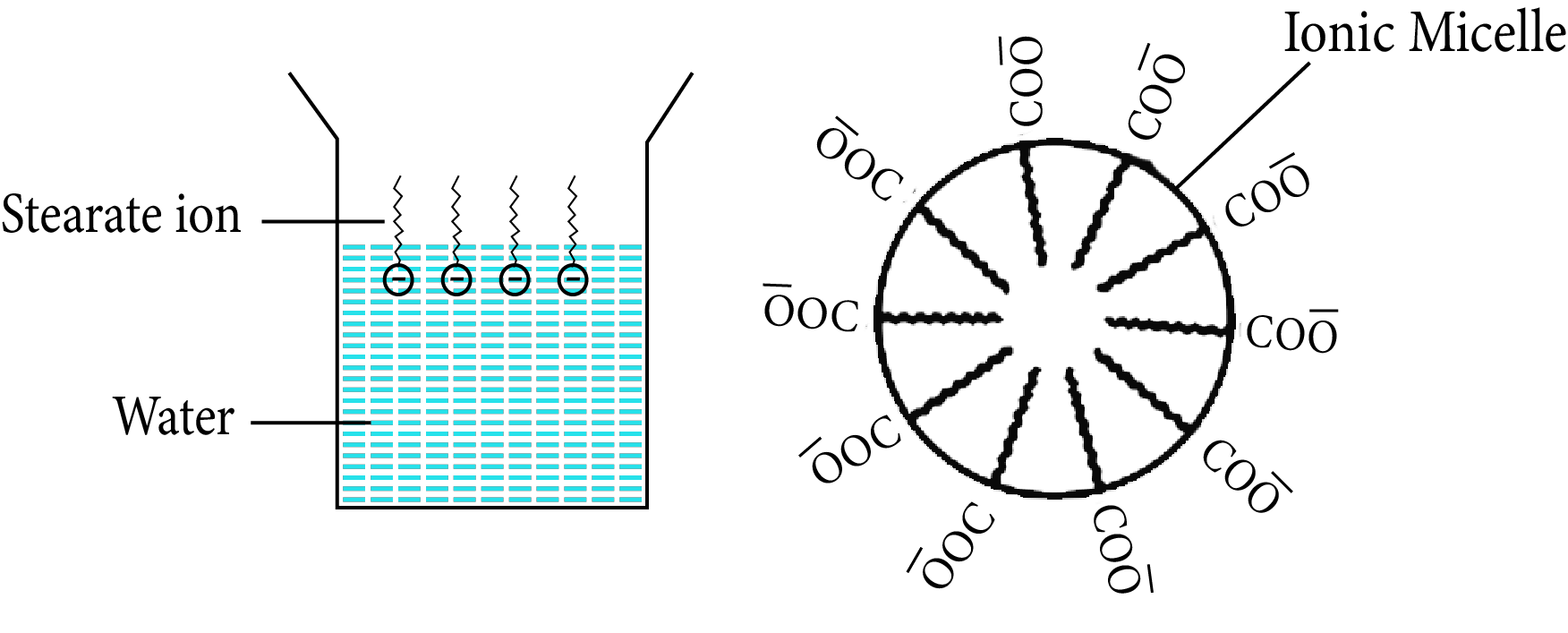
Associated Colloids : There are several compounds that are associated with colloids. At low concentrations, they behave like regular strong electrolytes, but at larger concentrations, they exhibit colloidal behaviour due to the colloidal nature of the material. Micelles are a type of the associated colloids. Micelles are formed only above a certain temperature. Temperatures above a certain point are referred to as Kraft temperatures (Tk). Critical micelle concentration is a term used to describe the concentration at which a micelle formation takes place (CMC).
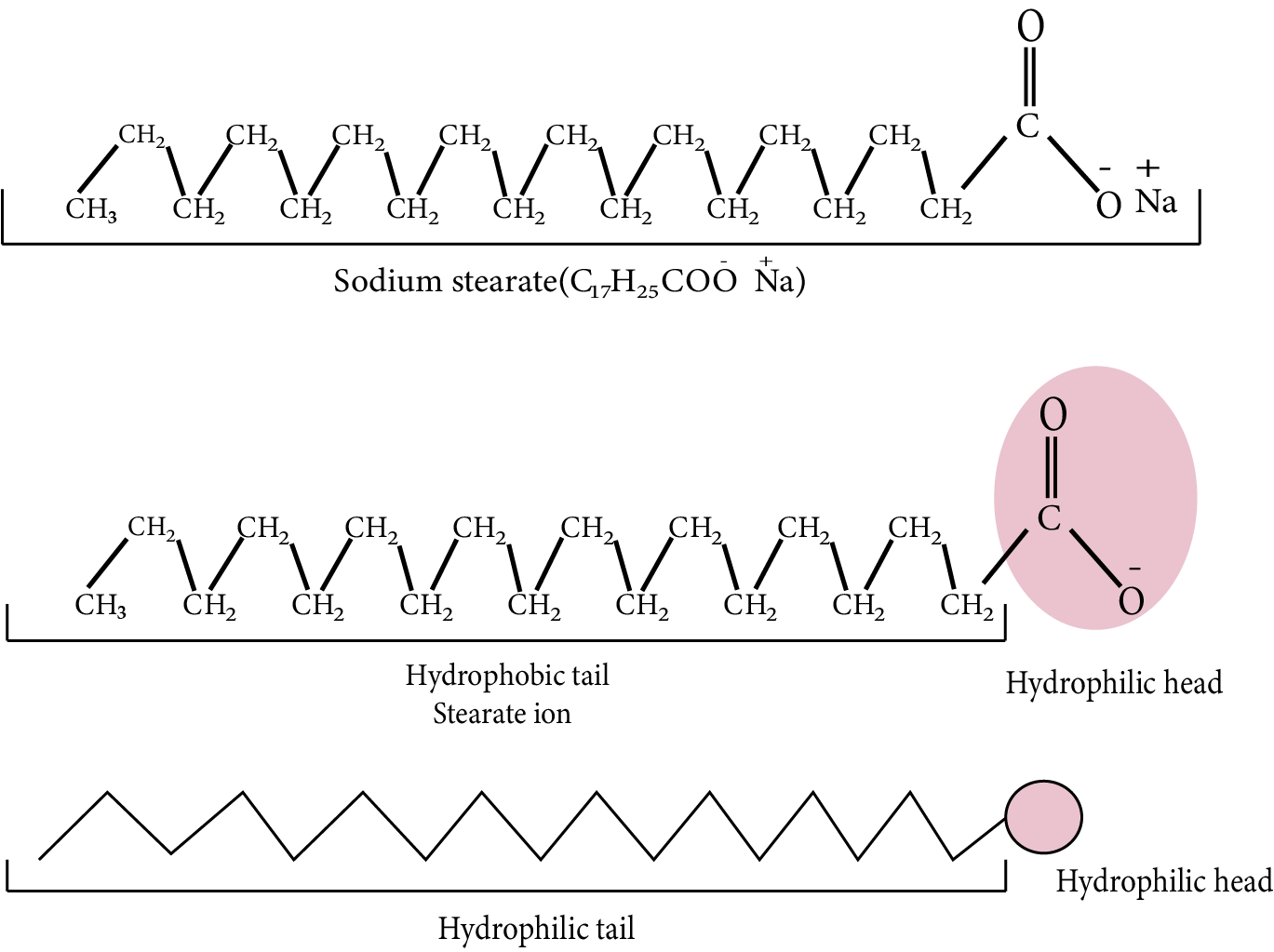
Mechanism of Micelle Formation
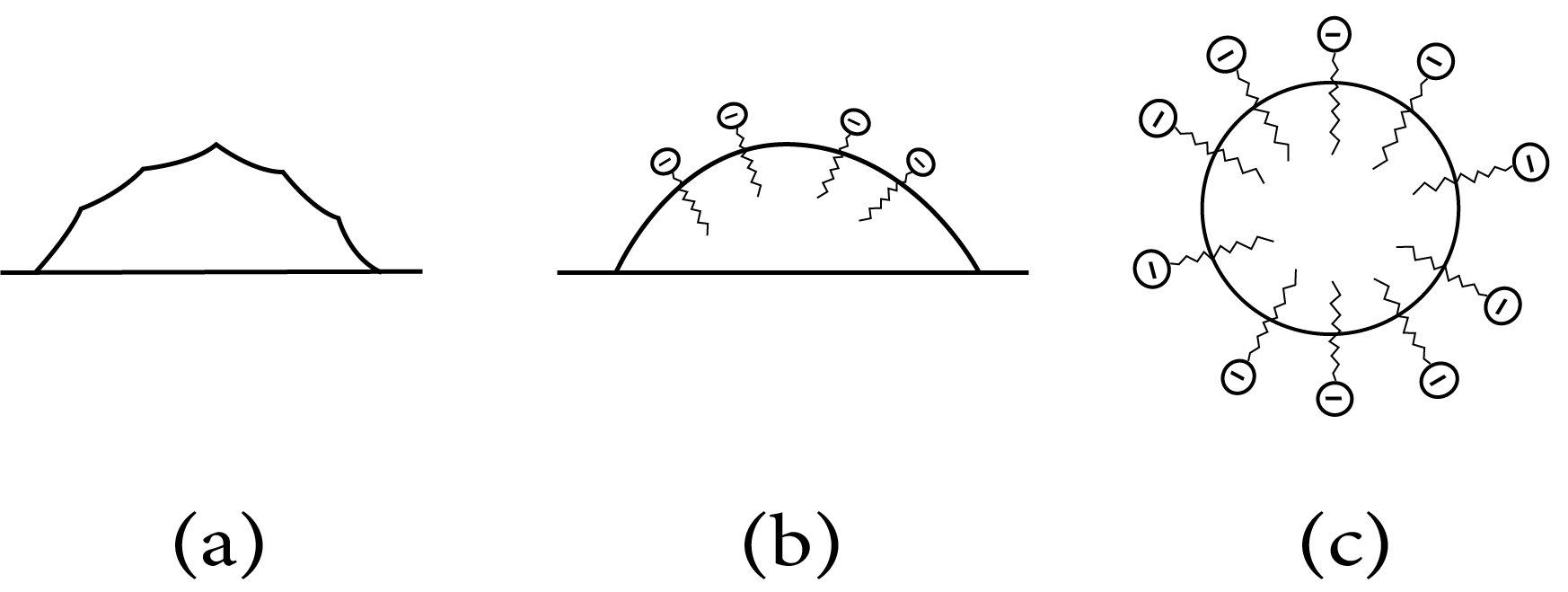
Soap is made up of sodium or potassium salt of a higher fatty acid as example- sodium stearate CH3 (CH2 )16COO–Na+ (e.g., sodium stearate CH3 (CH2 )16COO–Na+ , which is a key component of many bar soaps).It dissociates into RCOO– and Na+ ions when dissolved in water. The RCOO– ions, on the other hand, are made up of two parts: a hydrophobic (water repelling) long hydrocarbon chain R (also known as nonpolar 'tail') and a hydrophilic polar group COO– (also known as polar-ionic 'head') (water loving).
As a result, the RCOO– ions are present on the surface with their COO–groups in water, while the hydrocarbon chains R remain at the surface. However, at crucial micelle concentrations, the anions are drawn into the solution's bulk and aggregate to form the micelle.
Cleansing Action of Soaps
Soap has a cleaning effect because soap molecules produce micelles around them, the oil droplet in such a way that hydrophobic molecules are attracted to it. The oil droplet contains some of the stearate ions. The hydrophilic component of the grease protrudes and the droplet resembles bristles. Since the polar vortex, Water, the oil droplet, and groups can interact and is currently being dragged in by stearate ions and water and washed away from the filthy surface Thus, Emulsification and washing away are aided by soap.The sheath is negatively charged encircles the globules, preventing them from escaping clumping together and creating aggregations.
(d) - Preparation of Colloids
i) Chemical Techniques
Chemical reactions that result in the creation of molecules, such as double decomposition, oxidation, reduction, or hydrolysis, can be used to make colloidal dispersions.
ii) Bredig's Arc Technique or Electrical Disintegration
Both dispersion and condensation are involved in this process. This process can be used to make colloidal sols of metals such as gold, silver, platinum, and others. An electric arc is formed between metal electrodes immersed in the dispersion liquid in this process. The extreme heat causes the metal to vapourize, which then condenses into colloidal particles.
iii) Peptization
The process of turning a precipitate into colloidal sol by shaking it with dispersion medium in the presence of a little amount of electrolyte is known as peptidization. Peptizing agent is the electrolyte utilised for this purpose. In general, this procedure is used to turn a freshly generated precipitate into a colloidal sol.
The precipitate adsorbs one of the electrolyte's ions on its surface during peptization. Precipitates develop a positive or negative charge as a result, and eventually break up into smaller particles the size of a colloid.
(e) - Purification of Colloidal Solutions
Purification of colloidal solution is the process of lowering the number of contaminants to a required minimum. The following methods are used to purify colloidal solution
(i) Dialysis:
Dialysis is a process that involves diffusing a dissolved substance from a colloidal solution through a suitable membrane. The membrane can be utilised for dialysis because particles (ions or smaller molecules) in a real solution can flow through an animal membrane (bladder), parchment paper, or cellophane sheet but not colloidal particles. Dialyzer is the name of the device that is used for this purpose.
(ii) Electro-dialysis:
The dialysis procedure is normally rather slow. If the sole dissolved substance in the impure colloidal solution is an electrolyte, it can be made quicker by introducing an electric field. The procedure is then referred to as electrodialysis.
(iii) Ultrafiltration:
Ultrafiltration is the separation of colloidal particles from the solvent and soluble solutes in a colloidal solution using specially constructed filters that are impermeable to all substances except colloidal particles. Because the holes in regular filter paper are too large, colloidal particles can pass through.
(f) - Properties of Colloidal Solutions
Different properties exhibited by the colloidal solutions are described below:
(i) Colligative Properties:
Because colloidal particles are larger aggregates, the number of particles in a colloidal solution is lower than in a true solution. As a result, when compared to genuine solutions at the same concentrations, the values of colligative properties (osmotic pressure, reduction in vapour pressure, depression in freezing point, and elevation in boiling point) are of minor order.
(ii) Tyndall Effect:
When a homogeneous solution in the dark is examined in the direction of light, it appears clear; when observed at right angles to the direction of light, it appears perfectly dark. Colloidal solutions seen in the same way may look clear or translucent to transmitted light, but when viewed at right angles to the passage of light, i.eThe route of the beam is lighted by a bluish light, they show a mild to intense opalescence. The Faraday effect was first noticed by Faraday and later investigated in depth by Tyndall, and it is now known as the Tyndall effect. Tyndall cone refers to the bright light cone.
(iii) Colour:
The wavelength of light scattered by the dispersed particles determines the colour of colloidal solution. The size and type of the particles also influence the wavelength of light.
(iv) Brownian Motion:
When colloidal solutions are seen via a strong ultramicroscope, the colloidal particles appear to be moving in a zig-zag pattern throughout the field of view. The Brownian movement was named after the British botanist Robert Brown, who first noticed it. This motion is independent of the colloid's nature, although it is influenced by particle size and solution viscosity. The faster the motion, the smaller the size and the lower the viscosity.
(v) Colloidal Particle Charge:
Colloidal particles have an electric charge. The nature of this charge is the same for all particles in a colloidal solution, and it can be positive or negative.
(vi) Electrophoresis:
An electrophoresis experiment confirms the presence of charge on colloidal particles. Colloidal particles travel towards one of two platinum electrodes dipping in a colloidal fluid when an electric potential is put across them. Electrophoresis is the movement of colloidal particles under the influence of an electric potential.
5. Emulsions
These are colloidal liquid-liquid systems, in which finely divided droplets are dispersed in another liquid. Shaking a mixture of two immiscible or slightly miscible liquids produces an emulsion, which is a coarse dispersion of one liquid in the other. Water is usually one of the two liquids.
Emulsions are divided into two categories.
Water distributed in oil (W/O type) and oil dispersed in water (O/W type).
Water serves as a dispersion medium in the first system. Milk and disappearing cream are examples of this sort of emulsion.
Liquid fat is distributed in water in milk. Oil is used as a dispersion medium in the second system.
Butter and cream are common examples of this category.
Oil-in-water emulsions are unstable, and on standing, they can split into two layers. An emulsion is usually stabilised with the addition of a third component termed an emulsifying agent. Between suspended particles and the medium, the emulsifying agent generates an interfacial film.
Proteins, gums, natural and synthetic soaps, and other emulsifying agents are used in O/W emulsions, while heavy metal salts of fatty acids, long chain alcohols, lampblack, and other emulsifying agents are used in W/O emulsions.
Any amount of dispersion medium can be used to dilute emulsions. When blended, the dispersed liquid, on the other hand, creates a separate layer. Electrolytes can precipitate droplets in emulsions because they are generally negatively charged. They also demonstrate Brownian mobility and Tyndall efficiency. Butter is a good example of this kind.
6. Colloids Around Us
The following are some intriguing and significant colloidal examples:
The sky is blue because dust particles and water are suspended in the air. The sky appears blue to us because of the scattering of blue light that reaches our eyes.
Fog, mist, and rain: When a vast amount of dusty air is released into the atmosphere.When a particle is cooled below its dew point, the moisture in the air evaporates.
Food items: Milk, butter, halwa, ice creams, fruit juices, and other foods are all colloids in some way.
Blood, on the other hand, is a colloidal solution of an albuminoid material. Due to the styptic activity of alum and ferric chloride solution.Blood coagulates, forming a clot that prevents future bleeding.
Soils: Fertile soils are colloidal in nature, with humus serving as a binding agent, a colloid that protects. Soils adsorb because of their colloidal composition. Moisture and nutrient-rich materials are required.
Applications of Colloids
(i) Smoke electrical precipitation:
Smoke is a colloidal solution of carbon monoxide.
In the air, there are solid particles such as carbon, arsenic compounds, dust, and so on. Before it exits the chimney, the smoke passes via this passage a chamber housing plates with a charge that is the polar opposite of the one carried by the particles of smoke When these particles come into contact with them, the particles and Plates get precipitated when their charge is lost. Thus, the particles take a seat on the chamber's floor. The precipitator's name is Precipitator Cottrell .
(ii) Drinking Water Purification:
The water obtained from suspended contaminants are common in natural sources to coagulate the suspended matter, and alum is added to the water. Impurities are removed from the water, making it safe to drink.
(iii) Photographic Plates and Films:
Photographic plates and films are made by layering a light-sensitive silver bromide emulsion in gelatin over glass plates or celluloid films.
(iv) Rubber Industry:
Latex is a colloidal solution comprising negatively charged rubber particles. Latex coagulation is used to make rubber.
(v) Industrial Products:
Paints, inks, synthetic polymers, rubber, graphite lubricants, cement, and other industrial goods are all colloidal solutions.
Solved Examples from the Chapter
Example 1: Differentiate between the definitions of adsorption and absorption. Give one example from each category.
Solution: Adsorption is the process of a solid (or a liquid) attracting and keeping the molecules of a substance on its surface, resulting in a larger concentration of the molecules on the surface.
Adsorption is not the same as absorption. The material is equally distributed throughout the body of a solid or a liquid during absorption.
Key point to remember: Main difference between the absorption and adsorption on the basis of the concentration.
Example 2: What are emulsions, exactly? What are the many types? Give an illustration of each category.
Solution: Emulsions: It's a colloidal system in which the dispersed phase and the dispersion medium are both liquids, such as milk, which is made up of minute drops of liquid fat scattered in water.
Emulsions come in a variety of forms.
Oil-in-water type, in which small droplets of oil are spread in water, such as milk, cod liver oil, and so on.
Water droplets are scattered in an oil medium, such as butter, in the water-in-oil type.
Key point to remember: Definition of the emulsion and its two types oil in water and water in oil type and their examples based on their properties.
Solved Examples from Previous Year Questions
Question 1: Which one of the following characteristics is not correct for physical adsorption?
(a) Adsorption on solids is reversible
(b) Adsorption increases with increase in temperature
(c) Adsorption is spontaneous
(d) Both enthalpy and entropy of adsorption are negative.
Solution: There is always a decrease in surface energy which appears as heat when adsorption happens. So adsorption always takes place with the evolution of heat. Since the adsorption process is exothermic, the physical adsorption occurs readily at low temperatures and decreases with increasing temperature.
Hence option (b) is the answer.
Question 2: The aerosol is kind of colloid in which
(a) Gas is dispersed in liquid
(b) Liquid is dispersed in water
(c) Solid is dispersed in gas
(d) Gas is dispersed in solid.
Solution: The dispersion medium is gas in aerosol.
Hence option (b) is the answer.
Question 3: Peptization is a
(a) Process of converting a colloidal solution into precipitate
(b) Process of converting soluble particles to form colloidal solution
(c) Process of bringing colloidal molecule into solution
(d) Process of converting precipitate into colloidal solution.
Solution: Peptization is the process of converting precipitate into a colloidal solution.
Hence option (d) is the answer.
Practice Questions
Question 1: An example of solid sol is-
(a) Butter
(b) Use paint
(c) Cream for hair
(d) Precious stones
Solution: Solid sol is a colloid in which one solid is distributed in another continuous solid. A solid sol is something like a gemstone. As a result, option (d) is the correct answer.
Question 2: The correct option among the following is
(a) Colloidal particles in lyophobic sols can be precipitated by electrophoresis
(b) Colloidal medicines are more effective because they have small surface area
(c) Addition of alum to water makes it unfit for drinking
(d) Brownian motion in colloidal solution is faster if the viscosity of the solution is very high.
Answer: Electrophoresis is a technique that uses an electrical field to separate charged particles in a fluid. Electrophoresis can be used to precipitate colloidal particles in lyophobic sols. As a result, option (a) is the correct answer.
Conclusion
Adsorption is the phenomenon of a substance's molecules attracting and keeping themselves on the surface of a solid, resulting in a larger concentration on the surface than in the bulk. Adsorbate is the substance that is adsorbed, and adsorbent is the substance on which adsorption occurs. Adsorbate is kept to the adsorbent by weak van der Waals forces in physisorption, whereas adsorbate is attached to the adsorbent by a strong chemical bond in chemisorption. Gases adsorb on almost all solids. The amount of gas adsorption on a solid is determined by the nature of the gas, the nature of the solid, the solid's surface area, the gas's pressure, and the gas's temperature. In this chapter we have discussed what is surface chemistry and adsorption.
NEET Chapter Page - Surface Chemistry

 Share
ShareFAQs on NEET Chapter Page - Surface Chemistry
1. What applications does surface chemistry have?
The manufacture and operation of sorbents, solid reactants, catalysts for the synthesis of clean fuels and chemicals, pollution cleaning, photocatalysts, fuel cells, and batteries all rely on surface chemistry.
2. In surface chemistry, what is adsorption?
Adsorption is a mass transfer mechanism that occurs when gases or solutes adhere to solid or liquid surfaces. Due to imbalanced forces, the molecules or atoms on the solid surface have leftover surface energy, which is referred to as adsorption.
3. In surface chemistry, what is an emulsion?
A colloidal dispersion in which both the dispersed phase and the dispersion medium are liquids is known as an emulsion.




















 Watch Video
Watch Video


